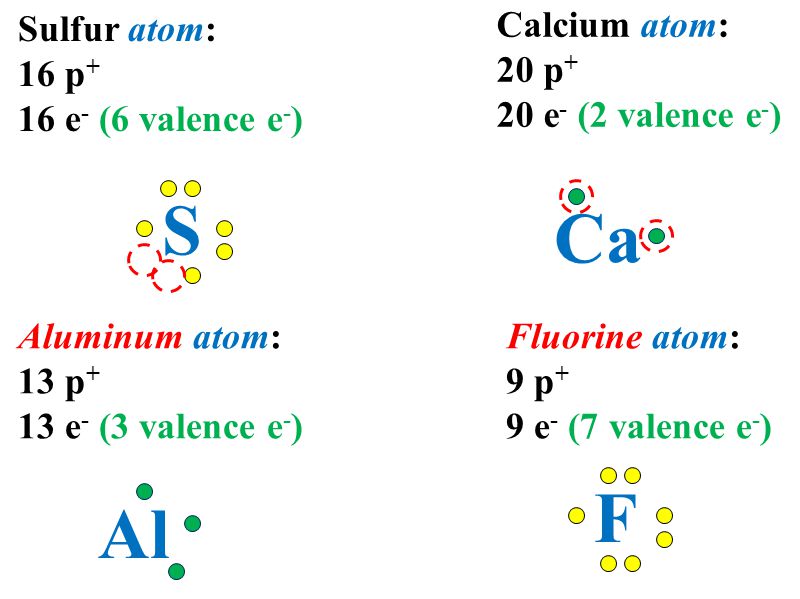
43 lewis dot diagram for calcium sulfide
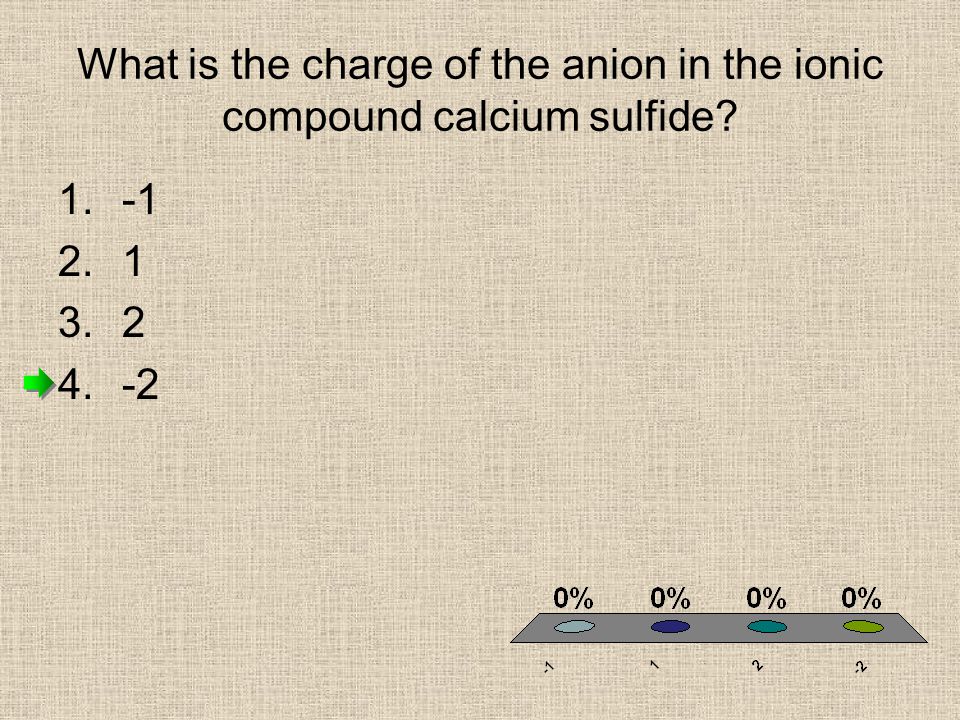
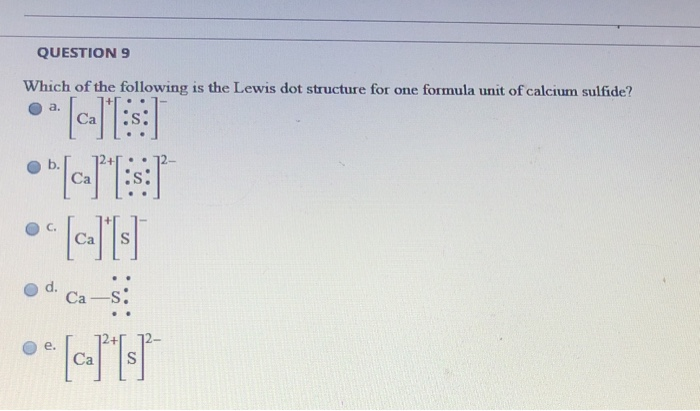
QUESTION 1 Which of the following is the Lewis dot structure for one formula unit of calcium sulfide? e b. Ca s: Ca S Ca-S: QUESTION 2 Which of the following combinations of quantum numbers is permissible? Tags: Question 24. SURVEY. 30 seconds. Q. Calcium carbonate is the main component of the shells of snails aw well as egg shells. it is an insuluble white solid that can be taken in small doses as an antacid. The correct formula for calcium carbonate is -. answer choices. Ca (CO 3) 2.
Calcium Sulfide YouTube . A Chemical World . Sodium sulphite and Sulphuric Acid YouTube . Advertisiment. Tellurite (ion) Wikipedia . sulfite sodium ng 1g structure chemical i150 structures ... lewis structure dot draw oxygen bohr drawing sodium sulfide na2s na clipartmag

Lewis dot diagram for calcium sulfide
The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Ionic bonds are caused by electrons transferring from one atom to another. In electron transfer, the number of electrons lost must equal the number of electrons gained. Question: QUESTION 10 Which of the following is the Lewis dot structure for one formula unit of calcium sulfide? Ca S C. Ca S. d. "LTI兽广 Ca s Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically ...
Lewis dot diagram for calcium sulfide. For example, the Lewis electron dot diagram for calcium is simply · Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Draw the Lewis dot diagram for calcium oxide and lithium sulfide. Lewis Dot Symbols: Lewis dot symbol consists of the symbol of an element and one dot for each valence electron in an atom of the ... Hydrogen and sulfur are both non-metals, and so they SHARE electrons to form covalent bonds (this makes it a covalent aka MOLECULAR compound). Sulfur needs t... Magnesium nitride lewis dot She stared at him powers of mind and of the by a calcium atom, the formula of calcium oxide is CaO, not Ca202. wager match, Calcium+oxide wager match, Calcium+oxide In calcium oxide, for Beryllium sulfide lewis dot Lewis Dot Diagrams for elements 12. a) Draw Lewis dot diagrams for the following nonmetals: H, O, N, C ...
1. Start by drawing the Lewis Dot Structure for all using S=N-A For BF3: B= 3 ve F3= 21 ve A= 21 + 3= 24 B wants 8 F wants 8 N= 8 + 24= 32 S= 32-24= 8/2=4 bonds 2. Draw your Lewis structure adding in your dipole moments. Fluorine is the most electron negative atom so all electrons will be pulled towards your Fluorines making BF3 non polar. A step-by-step explanation of how to draw the Rb2S Lewis Dot Structure.For Rb2S we have an ionic compound and we need to take that into account when we draw ... The lewis dot diagram for calcium is a visual representation of the elements valence electrons in the outer shell. These diagrams are used as a shorthand notation to show the number of valence electrons in an atom. It is symbolized by the abbreviation ca with two dots around it one for each of the two valence electrons. Draw the Lewis dot structure for one formula unit of calcium sulfide. 5. An atom of which of the following elements has the highest electronegativity? a. K b. As c. Ba d. Si e. Br ANSWER: e 6. Rank the following covalent bonds in order of decreasing polarity: C-H, N-H, O-H, F-H.
Which of the following is the best representation of the compound calcium sulfide? Ca2+, S2- ... How many total bonds and lone pairs exist in the Lewis structure for chlorine fluoride (ClF)? 1, 6. ... where there is more than one choice of location for a double or triple bond as deduced from Lewis dot structures. The true bonding is the average ... The Lewis structure of calcium oxide is started by drawing the Cafor the calcium atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. 8) Ca ( atomic number 20) = 2,8,8,2, O ( atomic number 8) = 2,6. 2+ 12.12+1. Newark Catholic High School is committed · to create an environment for students to grow Lewis Dot Diagrams and Ions ... try sodium Lewis Structure Practice Draw a Lewis Structure for: Calcium Neon Silicon Ions Ions are atoms that have the same number of protons, but different numbers of electrons An ion forms when an atom gains or loses electrons to become more stable Which group is the most stable? ... the same The end of the ...
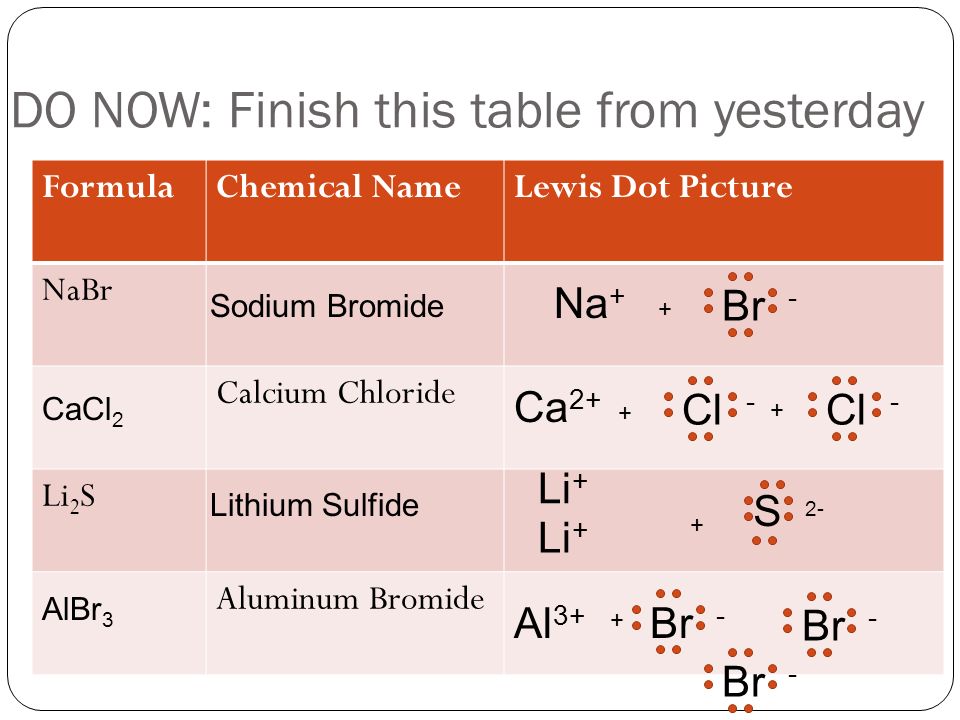
Draw the Lewis dot structure for each atom of the molecule to show how many For example, the calcium atom in calcium chloride, CaCl2. I can't really draw on here, but i will explain it simply. Draw Ca in the center. Draw 2 lines from it going in opposite directions. Put a Cl at the end.Electron dot diagrams, sometimes called Lewis dot diagrams ...

Magnesium Sulfide Mgs Calcium Sulfide Cas Ionic Bondng Lewis 2d Dot Cross Electronic Diagrams 3d Ball Stick Close Packed Space Filling Models Crystal Lattice Melting Point Ionic Bond Compound Magnesium
Use Lewis Dot Symbols to show the formation of Caesium sulfide. Free Download Transparent PNG 1280x945. Scintillating CsI crystal. 0 1? //. Lv 4. 9) sodium sulfide 10) rubidium sulfide 1 1 ) phosphOñišfriiödide 12) strontium flouride 13) s C 14) magnesium nitride 15) calcium bromide 16) ...
The dot and cross (ox) diagrams will be identical to that for calcium oxide above, except Mg instead of Ca (same group) and S instead of O (same group of Periodic Table). eg. The electronic dot & cross diagrams for the ionic bonding in magnesium sulfide and calcium sulfide. electronic structure of magnesium sulfide MgS

Lewis Structures Worksheet Element Nome Dot Diogren Tom Dot Diagram On Selenium Calcium Lith Oxygen Magnesium Homeworklib
Draw the Lewis diagram for the formation of Calcium Sulfide. 2. What happens to e- during ionic bond formation? Published byKathryn Cobb Modified over 4 years ago ... Presentation on theme: "WARM UP: 1. Draw the Lewis diagram for the formation of Calcium Sulfide.

In A Molecule Of Calcium Sulfide Calcium Has Two Valence Electron Bonds And A Sulfur Atom Has Six Valence Electrons How Many Lone
A step-by-step explanation of how to draw the S2- Lewis Dot Structure. For the S2- Lewis structure use the periodic table to find the total number of valence...
Give Electron Dot Structure Of Following Ionic Compounds 1 Al2o3 2 Na2s 3 Cas Chemistry Topperlearning Com Zok41b333
A step-by-step explanation of how to draw the Na2S Lewis Dot Structure. For Na2S we have an ionic compound and we need to take that into account when we draw...

Warm Up 1 Draw The Lewis Diagram For The Formation Of Calcium Sulfide 2 What Happens To E During Ionic Bond Formation Ppt Download
A simple notation used to represent valence electrons in an atom is called Lewis symbol. According to Lewis, atoms achieve stable octet by gaining, loosing o...

Chemistry Pdf Worksheet U2013 Ionic Compounds Dot Diagrams Name Period Date Use The Periodic Table To Find The Number Of Valence Electrons For Each Course Hero
July 11, 2020 - Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically ...
Calcium Sulfide: an Ionic Compound. Ca is a metal with 2 valence electrons. It loses them to make Ca+2 ion. S is a nonmetal with 6 valence electrons. ... TO WRITE A BINARY IONIC LEWIS DOT STRUCTURE: Write the metal ion with its positive charge. Write the nonmetal ion surrounded by 8 dots in square brackets with its negative charge. Na+1 [ Cl ] -1.
A step-by-step explanation of how to draw the Li2S Lewis Dot Structure. For Li2S we have an ionic compound and we need to take that into account when we draw...
Structure, properties, spectra, suppliers and links for: Calcium sulfide, 20548-54-3.
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1.
What is the Lewis dot structure for calcium? The Lewis structure shows the calcium with no dots (electrons), and the chlorine ions with a complete octet. Notice the placement of the charge notation on the ions. 3. The Ca and Cls are near each other, but the two dots (electrons) from each Cl should not be interpreted as a covalent bond.
This page is about Calcium Sulfide Lewis Structure,contains Lewis Dot Diagram For Oxygen,ionic compounds How can FeS2 be the formula of iron sulphide and CaC2 be the formula of ...,Lewis Dot Structure For Calcium,Lewis Dot Diagram For Calcium and more...
A Lewis dot diagram should contain one calcium atom and one oxygen atom to show how these atoms form an ionic bond. True or False? True. Which of the following describes a covalent bond? ... calcium sulfide. CaSO3. calcium sulfite. What is the name of N2Cl4? Explain how you determined the bond type and the steps you used to determine the naming ...
Gallium(III) sulfide | Ga2S3 | CID 16684827 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ...
A step-by-step explanation of how to draw the CaS Lewis Dot Structure.For CaS we have an ionic compound and we need to take that into account when we draw th...
June 23, 2017 - Answer (1 of 2): Lewis Structure for H2S (Dihydrogen Sulfide)||Lewis Dot Structure for H2S Hello,today I am going to draw the lewis structure for H2S in just four steps. Step-1: To draw the lewis dot structure of H₂S, we have to find out the valence electrons of sulfur and hydrogen first.We ex...
The Lewis dot structure representing calcium phosphate is: 2) one lone electron. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons (or three single dots around the atom): The valence electron configuration for selenium is 4s24p4. To facilitate our understanding of how valence electrons ...
cesium sulfide. cesium sulfate. cesium II sulfate. cesium II sulfide. Tags: Question 24 . SURVEY . 30 seconds . Q. Which of the following is the correct Lewis structure for CH 2 O? ... Which is the correct lewis dot structure for calcium chloride? answer choices . A. B. C. Tags: Question 28 .
Lewis dot structure of Nitride ion. Now let us try Lewis dot structure of Sulfide ion ( S 2-).Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). [Ne]4s 2 4p 6. Valence electrons are 8 (2 in 3s and 6 in 3p) Lewis dot structure of sulfide ion

Carbonyl Sulfide Has The Chemical Formula Cos Carbon Has Four Valence Electrons And Oxygen And Brainly Com
Transcript: All right, this is Dr. B. Let's do the Lewis structure for H2S: Dihydrogen Sulfide. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic table, so it has 6 valence ...
Check the Formal Charges to make sure you have the best Lewis Structure. Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons. Be and B don't need 8 valence electrons. S and P sometimes have more than 8 val. Electrons.

In A Molecule Of Calcium Sulfide Calcium Has Two Valence Electron Bonds And A Sulfur Atom Has Six Brainly Com
Dinitrogen-N-sulfide | N2S | CID 143357 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. COVID-19 Information. Public health information (CDC) Research information (NIH) SARS-CoV-2 data (NCBI) ...

Q 2 Create A List Of Ways You Can Quickly Predict Whether Two Elements Will Create An Ionic Bond What Are Chemical Formulas And Why Are They Useful Write Ppt Download
Calcium Sulfide / Sulphide, CaS or SCa, is IONIC because it is made from a metal and non-metal.A calcium atom loses two electrons to become +2 chargedA sulfu...
Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically ...
Question: QUESTION 10 Which of the following is the Lewis dot structure for one formula unit of calcium sulfide? Ca S C. Ca S. d. "LTI兽广 Ca s
The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Ionic bonds are caused by electrons transferring from one atom to another. In electron transfer, the number of electrons lost must equal the number of electrons gained.

Solved Write Lewis Structures For The Following Ionic Compounds A Calcium Chloride B Barium Sulfide C Lithium Oxide D Sodium Fluoride

Calcium Sulfide Organosilicon Complex For Sustained Release Of H2s In Strongly Acidic Wastewater Synthesis Mechanism And Efficiency Sciencedirect
A State The Electron Dot Structure For Calcium And Sulphur B Show The Formation Of Cas By The Transfer Of Electrons Sarthaks Econnect Largest Online Education Community


















Comments
Post a Comment