39 n2- molecular orbital diagram
N2 molecular orbital energy level diagram also has the following tags. The result is a slight change in the relative energies of the molecu... Molecular orbital diagrams say about the mixing of orbitals in a compound. Using a MO diagram, the bond order of a compound can be determined The two atomic orbitals will be placed side by side. The oxygen will remain the same in nitrous oxide as well. And you can form the molecular orbital by...
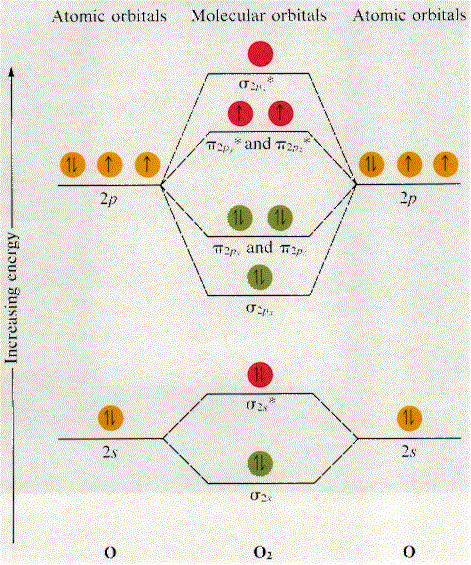
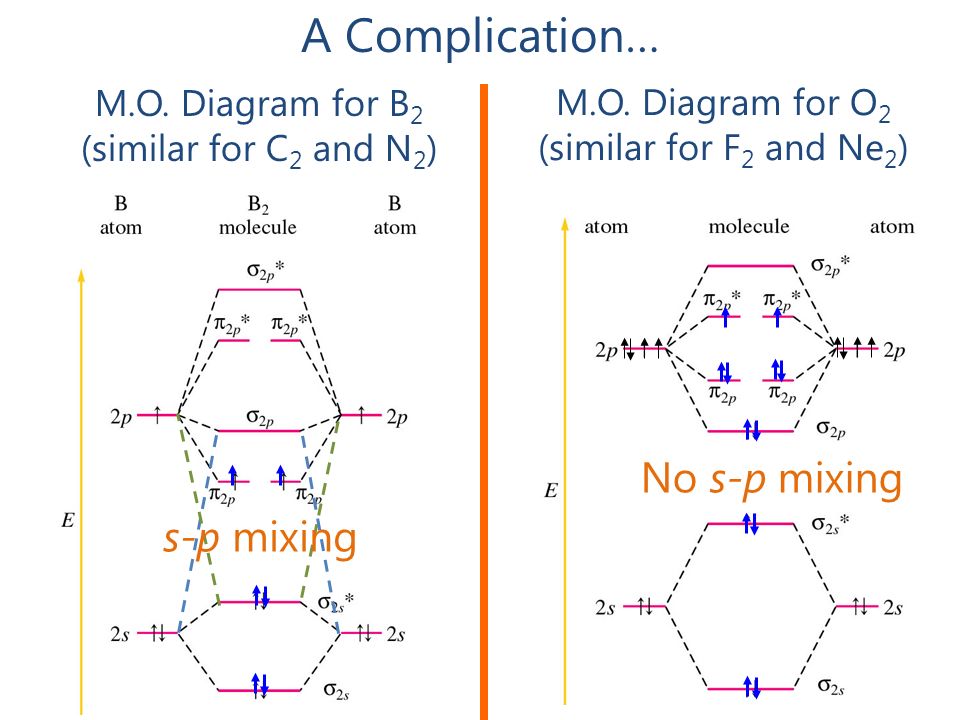
Indicate if it is diamagnetic or paramagnetic. In o 2 and f 2 there is a crossover of the sigma and the pi ortbials.
N2- molecular orbital diagram
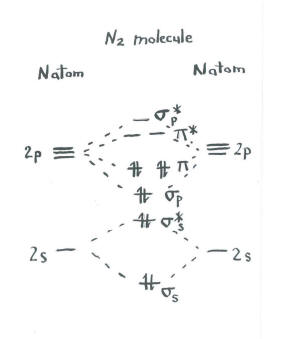
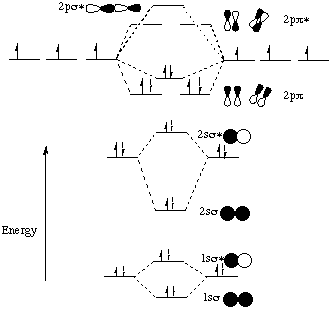
The other molecular orbital produced s h h shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint... Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this Molecular orbital mo diagram of n2 molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you sigma2s 2 sigma2s 2 pi2p 4 mo diagram for n2 molecular orbital there are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc.
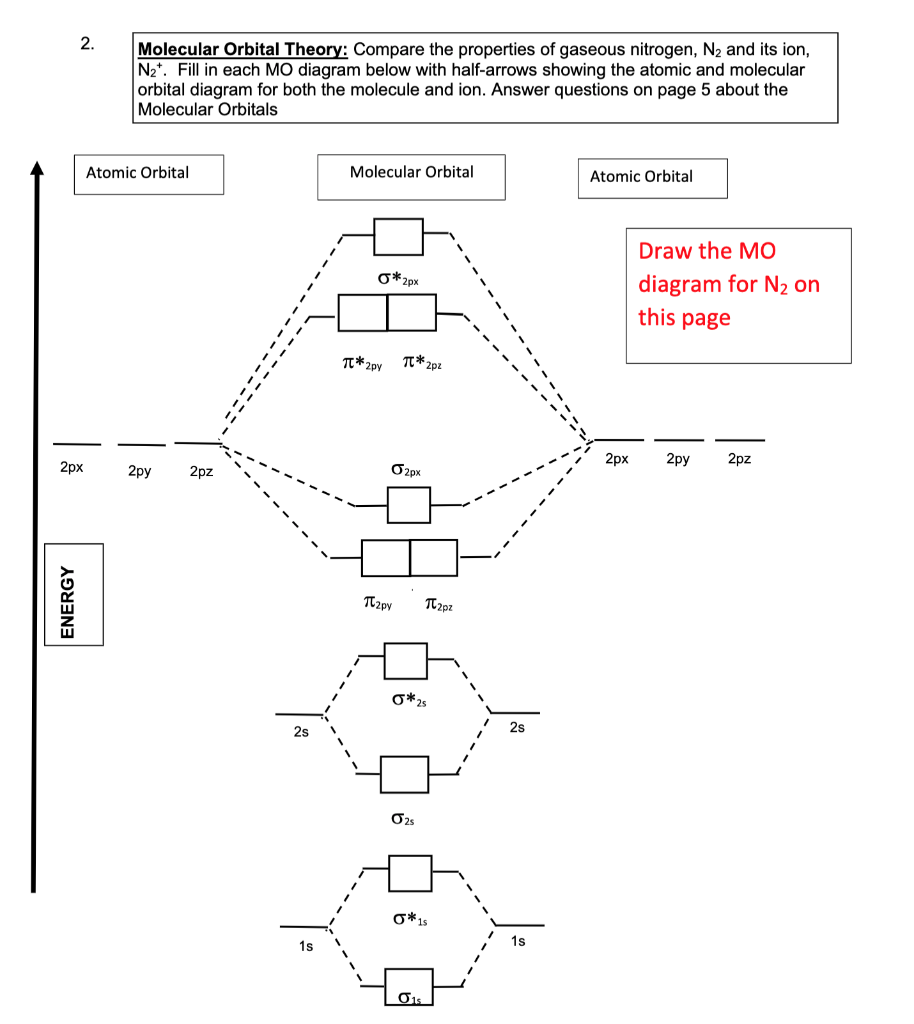
N2- molecular orbital diagram. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The orbital energies decrease across the period as the effective nuclear charge increases and atomic radius decreases. Between N2 and O2, the order of the orbitals changes. Whenever two orbitals interact to form molecular orbitals, they form a set of two molecular orbitals: one bonding orbital and one antibonding orbital. For example, here's the MO diagram for `N_2`. We know from the Lewis structure that `N_2` has a triple bond. This means that the bond order of `N_2... Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond... Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.
Interact and form molecular orbitals. Now we add the 10 electrons 5 from each nitrogen atom. Mo Diagram For Formation Of Ni... The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The orbital energies decrease across the period as the effective nuclear charge increases and atomic radius decreases. Between N2 and O2, the order of the orbitals changes. Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the This configuration accounts for the considerable strength of the bonding in N2 and consequently its ability to act as a diluent for the oxygen in the... Answer : According to the molecular orbital theory, the general molecular orbital configuration will be, As there are 7 electrons present in nitrogen. The bond order of is, 3. The molecular orbital diagram of are shown below.
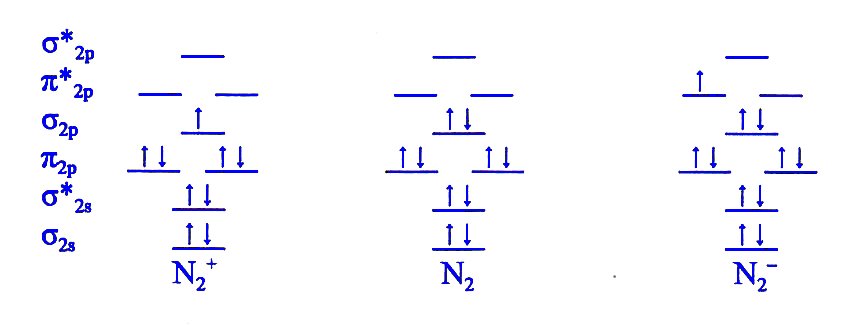
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The orbital energies decrease across the period as the effective nuclear charge increases and atomic radius decreases. Between N2 and O2, the order of the orbitals changes. Draw the Molecular Orbital Diagram of H2+, O2- and N2 molecules and calculate their bond orders as well as tell about their properties, whether they are paramagnetic or diamagnetic ? LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital. bond order (H2 molecule) =. Sigma (σ) bonding molecular orbital - Shared electron density is directly between the bonding atoms, along the bonding axis. A sigma bonds is always the first bond formed... Three filled bonding orbitals. Draw the molecular orbital diagram for n2 ion and calculate the bond order. Introduction To...
Solved Draw The Hybridized Molecular Orbital Picture For Molecular Nitrogen N2 Label Each Orbital E Sp2 Sp3 S And Show How They Overlap In Course Hero
Molecular Orbital Diagram For N 2. Molecule Enetsy Level Diagram including each atom's energy levels) Molecular orbital clectron. Molecular Orbital Diagrams - N2 - YouTube. inorganic chemistry - MO for $N_2^2-$ - Chemistry Stack ... (a) N atom orbitals and their linear combination to form ...
Will the MO diagram be the same as that of $\ce{N2}$ or not? Now note that even in this advanced molecular orbital theory a bunch of approximations is introduced, and the answer in general depends on at which level of theory calculations are done.
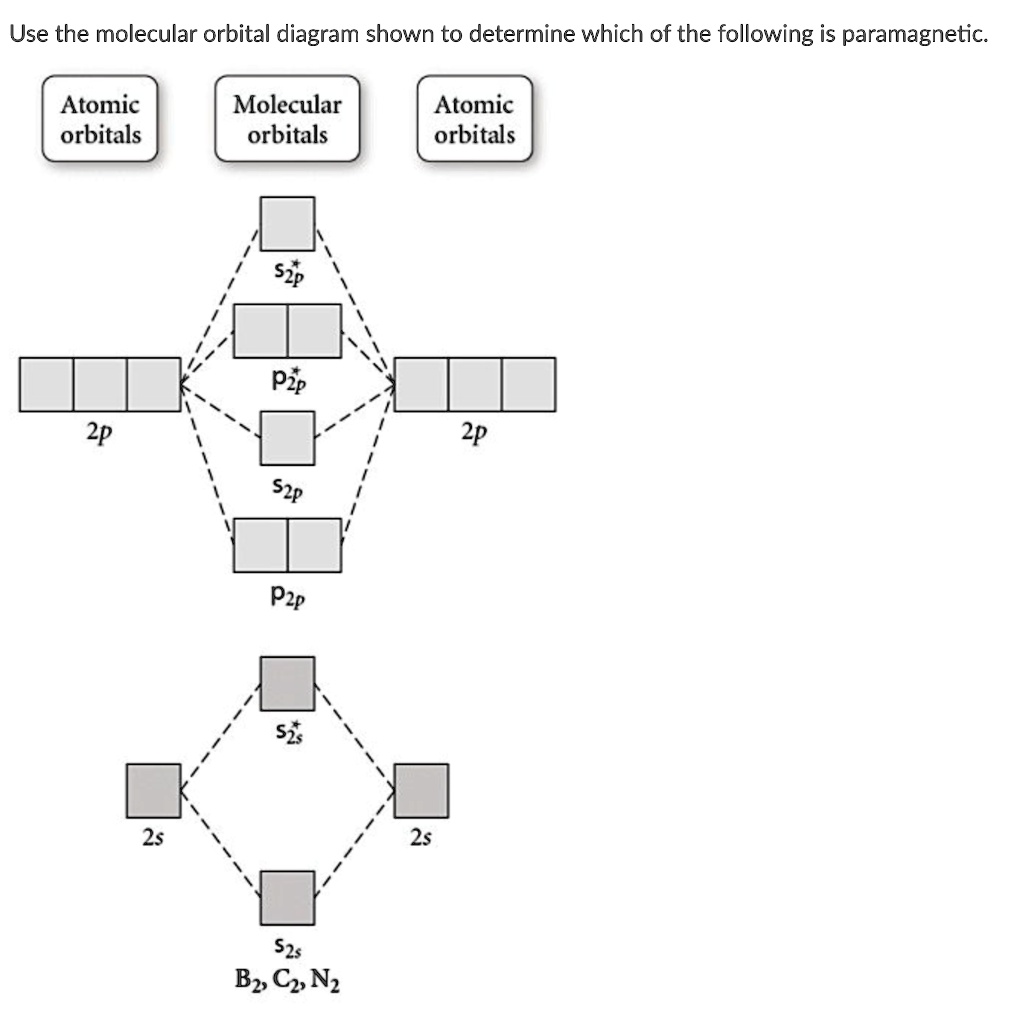
Solved Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic Atomic Orbitals Molecular Orbitals Atomic Orbitals S2p Pip 2p 2p S2p Pzp 2s 2s 52s Bz Cznz
N b 8 na 2. Draw the molecular orbital diagram for n2 ion and calculate the bond order. Orbitals What Is The Origin Of The...
Give The Molecular Orbital Energy Diagram Of N2 And O2 Write The Bond Order Of N2 And O2 Sarthaks Econnect Largest Online Education Community
molecular orbital diagram for N2. number of electrons in the sigma2p molecular orbital is. their molecular orbital diagrams are more symmetrical than those of homonuclear diatomic molecules. which of the following statements about nitrogen oxide, NO, is FALSE.
The other is for after nitrogen starting at oxygen. Diatomic molecules made up of two different atoms also have molecular orbital diagrams...
Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
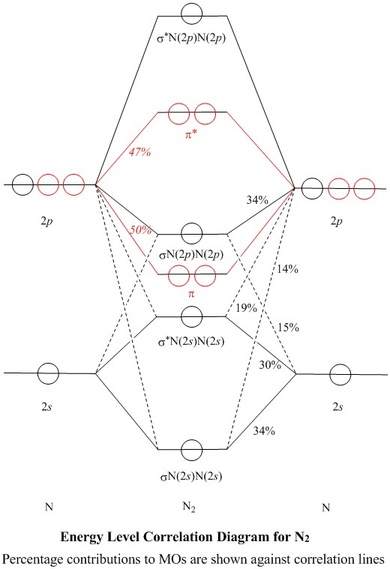
Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.

89 Chemical Bonding 36 Covalent Bonding 35 Molecular Orbital Theory 10 Nitrogen Molecule Madoverchemistry Com
You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also help us to find out the bond order of the Complete step by step answer: First let us understand the concept of molecular orbital theory. On a very general basis, electrons are not...
• Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Molecular orbital mo diagram of n2 molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you sigma2s 2 sigma2s 2 pi2p 4 mo diagram for n2 molecular orbital there are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc.
Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this
The other molecular orbital produced s h h shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint...

Write The Molecular Orbital Diagram Of N2 And Calculate Their Bond Order Chemistry Topperlearning Com Qbqjy

Write The Molecular Orbital Configuration And Energy Diagram For N2 N2 N2 Chemistry 12902653 Meritnation Com

















Comments
Post a Comment