40 pi molecular orbital diagram
molecular orbitals. 2.The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. 3.The additive overlap results in the bonding molecular orbital while the subtractive overlap results in the antibonding overlap. 4.The energy of bonding molecular orbitals is lower than their nonbonding counterparts ... Today, let's go through how to draw out the molecular orbitals of benzene.In the case of cyclic systems, the (n-1) rule fails.Instead of looking at these as ...
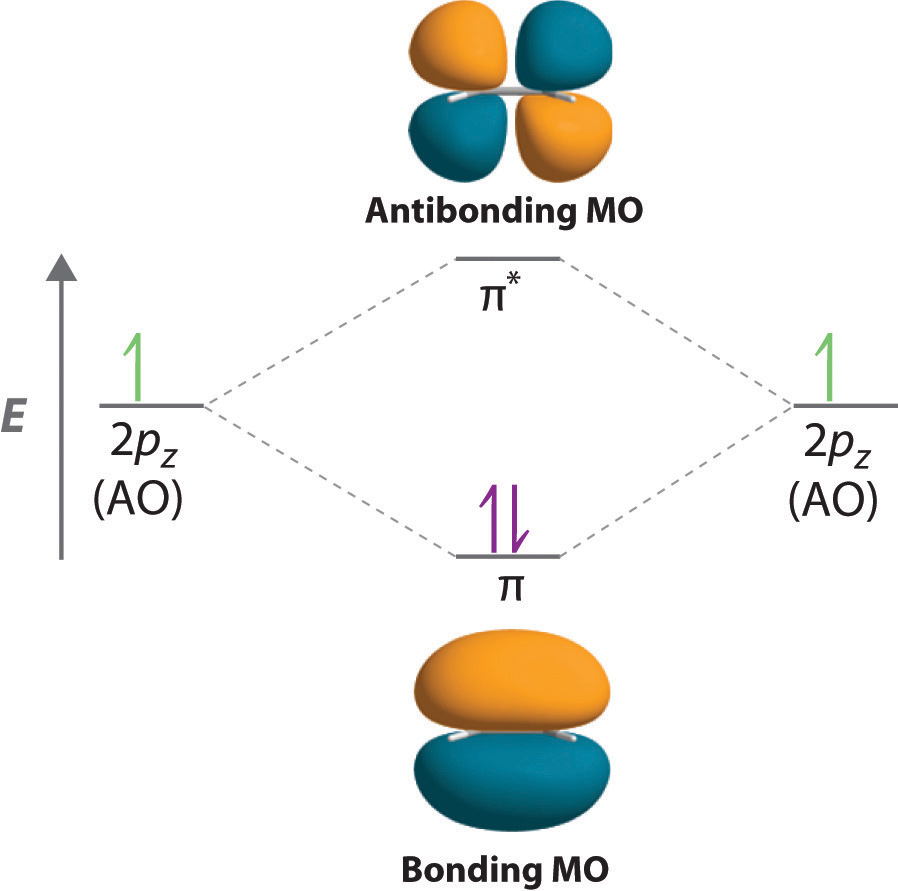
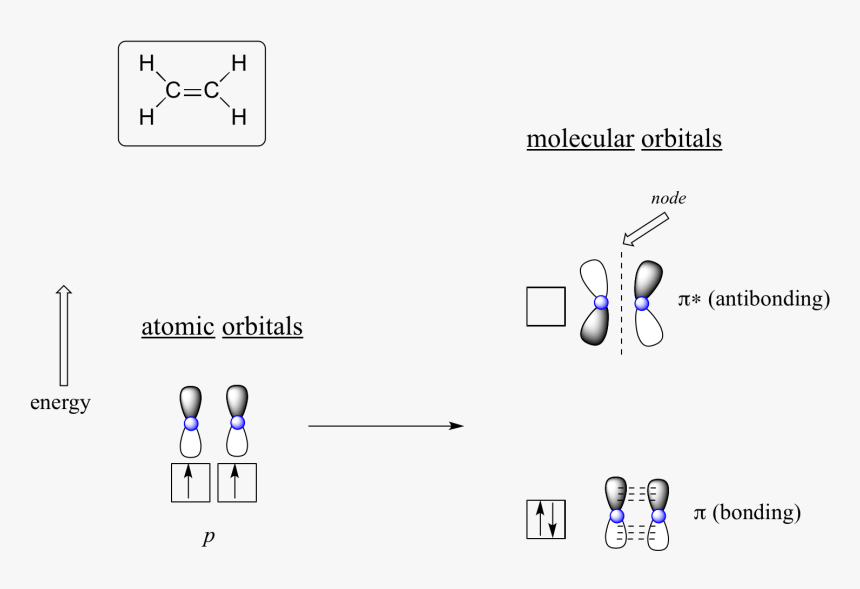
Pi Molecular Orbitals of Ethylene. In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals: ψ1 and ψ2*, (also referred to as π1 and π2*). ψ1 is a bonding molecular orbital, is occupied in the ground state, and is the ...
Pi molecular orbital diagram
The sum of these side-to-side interactions increases the electron probability in the region above and below a line connecting the nuclei, so it is a bonding molecular orbital that is called a pi (π) orbital (a bonding molecular orbital formed from the side-to-side interactions of two or more parallel np atomic orbitals). 00:26 Reducible representation for pi group orbitals03:33 Reduction of reducible representation13:20 Effect of each symmetry operation on representativ... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
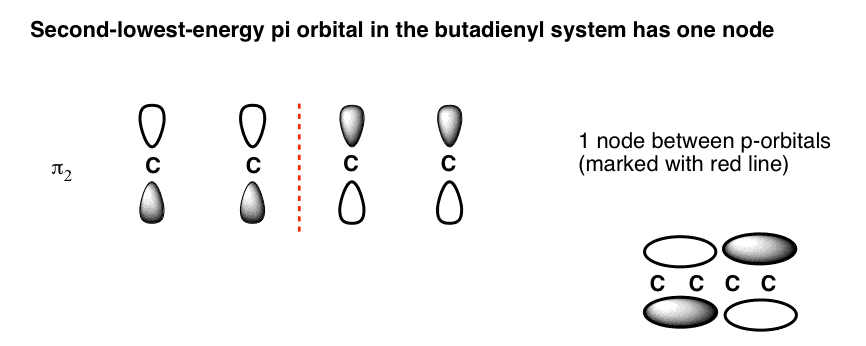
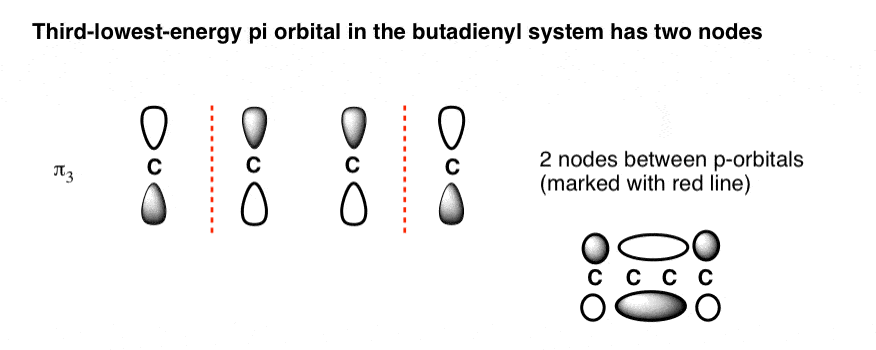
Pi molecular orbital diagram. Feb 28, 2017 · The Highest-Energy Molecular Orbital (π4) Has Three Nodes. The highest-energy molecular orbital is also very easy to draw. Just draw n (4 in our case) p orbitals and alternate the phases of each. This creates a pi system with three nodes (areas where the lobes change sign). We’ve drawn the nodes in as red dotted lines. Note: when the 3 p orbitals (x,y,z) interact, they form 4 pi bonds and 2 sigma bonds Heteronuclear Diatomic Molecules Because the electronegativity of the two atoms are unequal, the molecular ... Pi Molecular Orbitals of Ethene zIn chapter 1 we saw that the molecular orbitals of H2 are created by the combination of 1s orbitals. zThe in-phase combination gave the bonding orbital. zThe out-of-phase combination the anti-bonding orbital. zFor ethene, the σιγµα framework is created by the interaction of the sp2 hybrid orbitals of the C atoms and H1s Mar 04, 2021 · Describe the essential difference between a sigma and a pi molecular orbital. Define bond order, and state its significance. Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable.
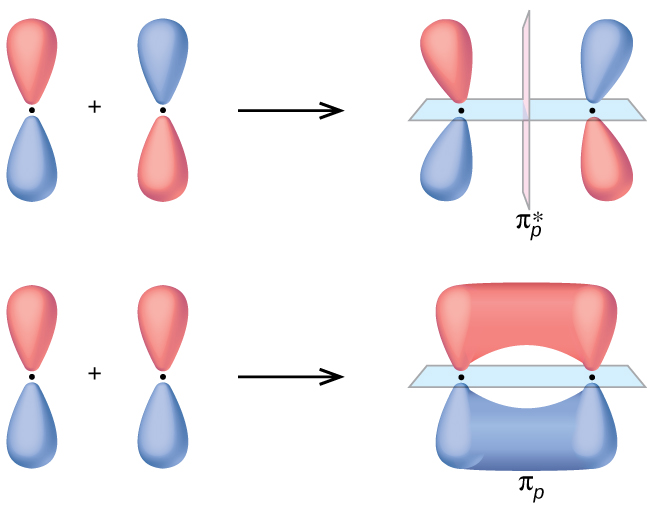
In both molecules the pi symmetry molecular orbitals are the same. The 2p x orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital in the xz plane. Perpendicular to these in the yz plane, the 2p y orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital. Here is the full molecular orbital diagram for N 2. Molecular Orbital (MO) Theory is the final theory pertaining to the bonding between molecules. In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals, molecular orbital theory visualizes bonding in relation to molecular orbitals, which are orbitals that surround the entire molecule. The purpose of MO theory is to fill in the gap for some behavior that ... Pi Molecular Orbitals of 1,3,5-Hexatriene. With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure. There are six adjacent carbon atoms involved in the pi ... The side-by-side overlap of two p orbitals gives rise to a pi ([latex]\pi[/latex]) bonding molecular orbital and a [latex]\pi[/latex]* antibonding molecular orbital, as shown in Figure 7.7.6. In valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p-[latex ...
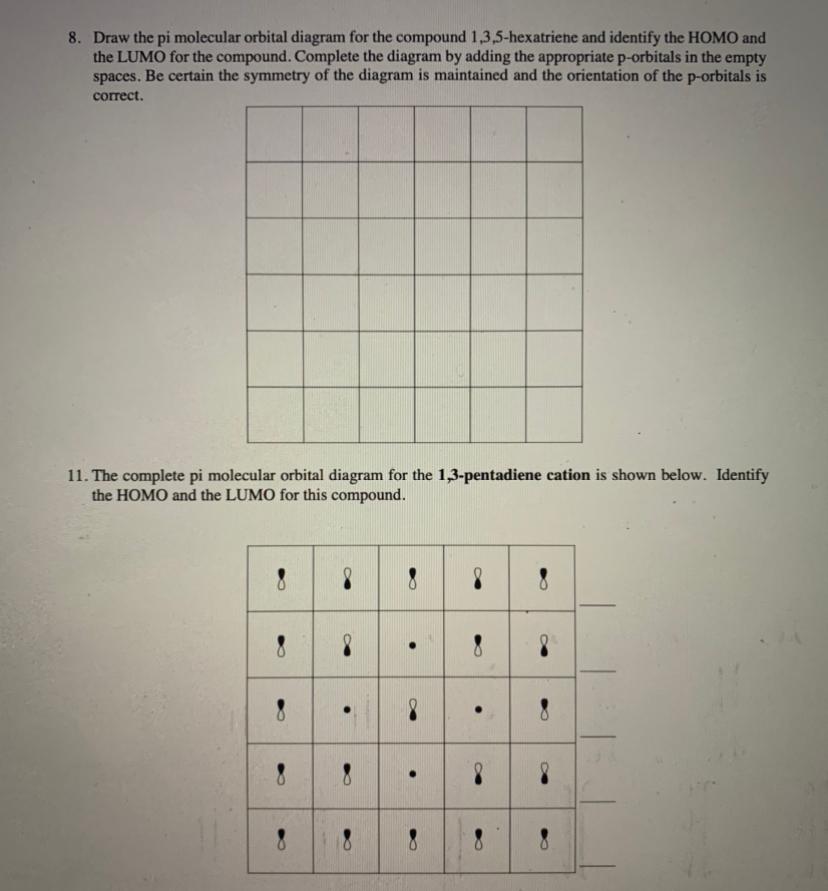
8 - Drawing Molecular Orbital Diagrams. Abstract (TL;DR) Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are ... On the other hand, the bonding molecular orbitals of t2g are higher in energy than all σ bonding molecular orbitals. The overall molecular orbital energy level diagram for this type of π-bonding in octahedral complexes can be shown as: Figure 21. The generation of π and σ-molecular orbitals in octahedral complexes. This video is explaining, the formation of Pi molecular orbitals of Hexatriene and Benzene. All the six electrons would be there in si1, si2 and si3 molecula... Question: 8. Draw the pi molecular orbital diagram for the compound 1,3,5-hexatriene and identify the HOMO and the LUMO for the compound. Complete the diagram by adding the appropriate p-orbitals in the empty spaces. Be certain the symmetry of the diagram is maintained and the orientation of the p-orbitals is correct. 11.
MOLECULAR ORBITALS. They result from combinations of orbitals between atoms as bonding takes place to form molecules. LEARNING OBJECTIVES To introduce the basic principles of molecular orbital theory and electronic geometry of molecules. ... sigma bond, and the bond formed by the p orbitals is called a pi bond. The process is shown below.
Chapter 1 Molecular Orbital Concepts A Concepts Of Mo Theory 1 Strong Covalent Bonds Consider The Pi Bond Of Ethene In Simple Molecular Orbital Terms The Qualitative Results Would Be The Same For Any Pi Or Sigma Bond Q The Overlap Of The Two
O3 Molecular Orbital Diagram. The ozone molecule's Lewis structure shows that even the preferred structure The pi molecular orbital energy diagram for ozone into which are distributed four . Please draw MO diagram for ozone (O3). I saw a pic for O3 where the 2s of O was interacting with both bonding and antibonding. Can you please draw and it.
Pi molecular orbitals of polyenes. Ask Question Asked 4 years, 5 months ago. Active 1 month ago. Viewed 2k times 11 1 $\begingroup$ As an organic chemist, I'm comfortable deriving the pi molecular orbitals of linearly conjugate systems to give the following result: In a qualitative sense, these molecular orbitals are easily arrived at. ...
Bonding in CH 4, built from 4 hydrogen 1s orbitals and 4 hybrid carbon sp 3 orbitals, has 8 molecular orbitals in total. 7. Bonding Pi Molecular Orbitals Form Through The Side-On Constructive Overlap Of p Orbitals. In this series of posts, we've been discussing Pi (π)bonding, which is the interaction (overlap) between two p orbitals.
The two 2p z orbitals overlap to create another pair of pi 2p and pi *2p molecular orbitals. The 2p z-2p z overlap is similar to the 2p y-2p y overlap because it is just the orbitals of the 2pz rotated 90 degrees about the axis. The new molecular orbitals have the same potential energies as those from the 2p y-2p y overlap.
The side-by-side overlap of two p orbitals gives rise to a pi (π) bonding molecular orbital and a π* antibonding molecular orbital, as shown in Figure 5. In valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p orbitals, with electron density on either ...
Individual atomic orbitals (AO) are arranged on the far left and far right of the diagram. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram. These MO overlap with either a sigma or pi bond and are designated in bonding, nonbonding, or antibonding orbitals with respect to their phases.
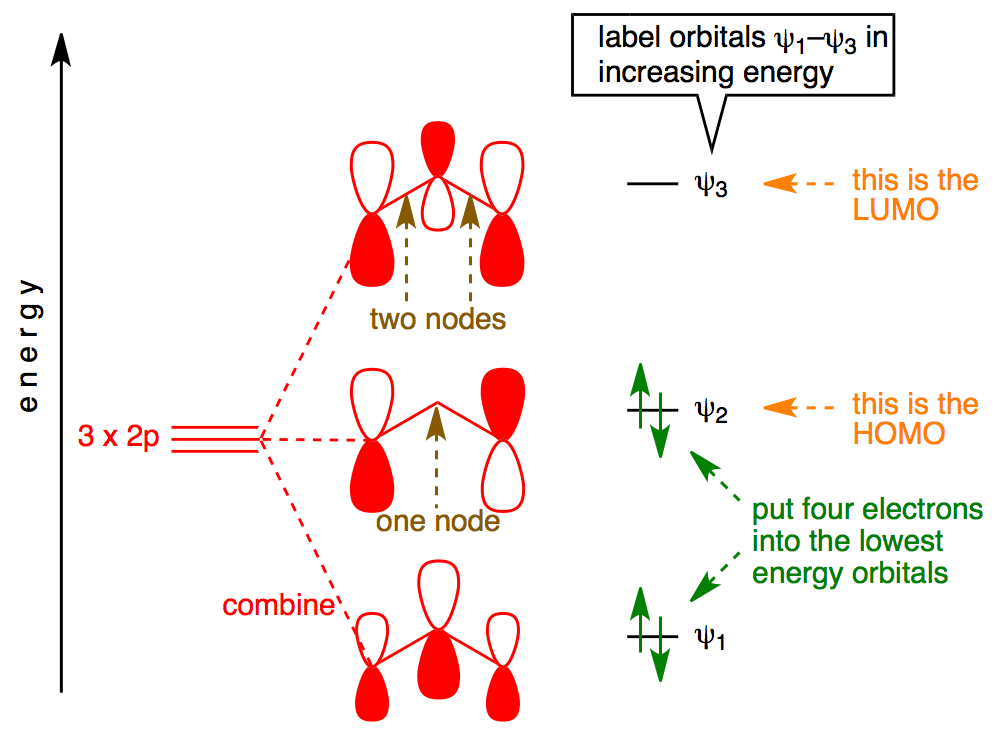
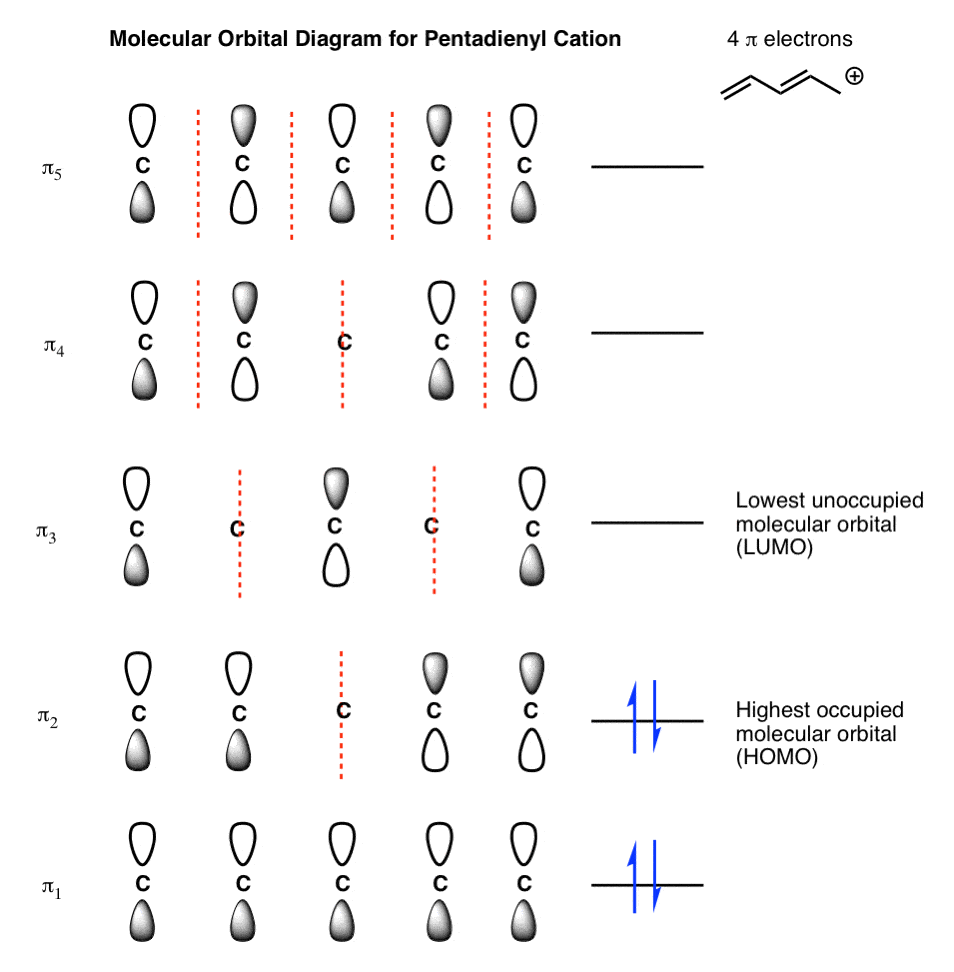
note that from our N pz orbitals we will obtain N π orbitals. Further, each carbon atom has one free valence electron to contribute, for a total of N electrons that will need to be accounted for (assuming the molecule is neutral). Accounting for spin, then, there will be N/2 occupied molecular orbitals and N/2 unoccupied ones. For the ground
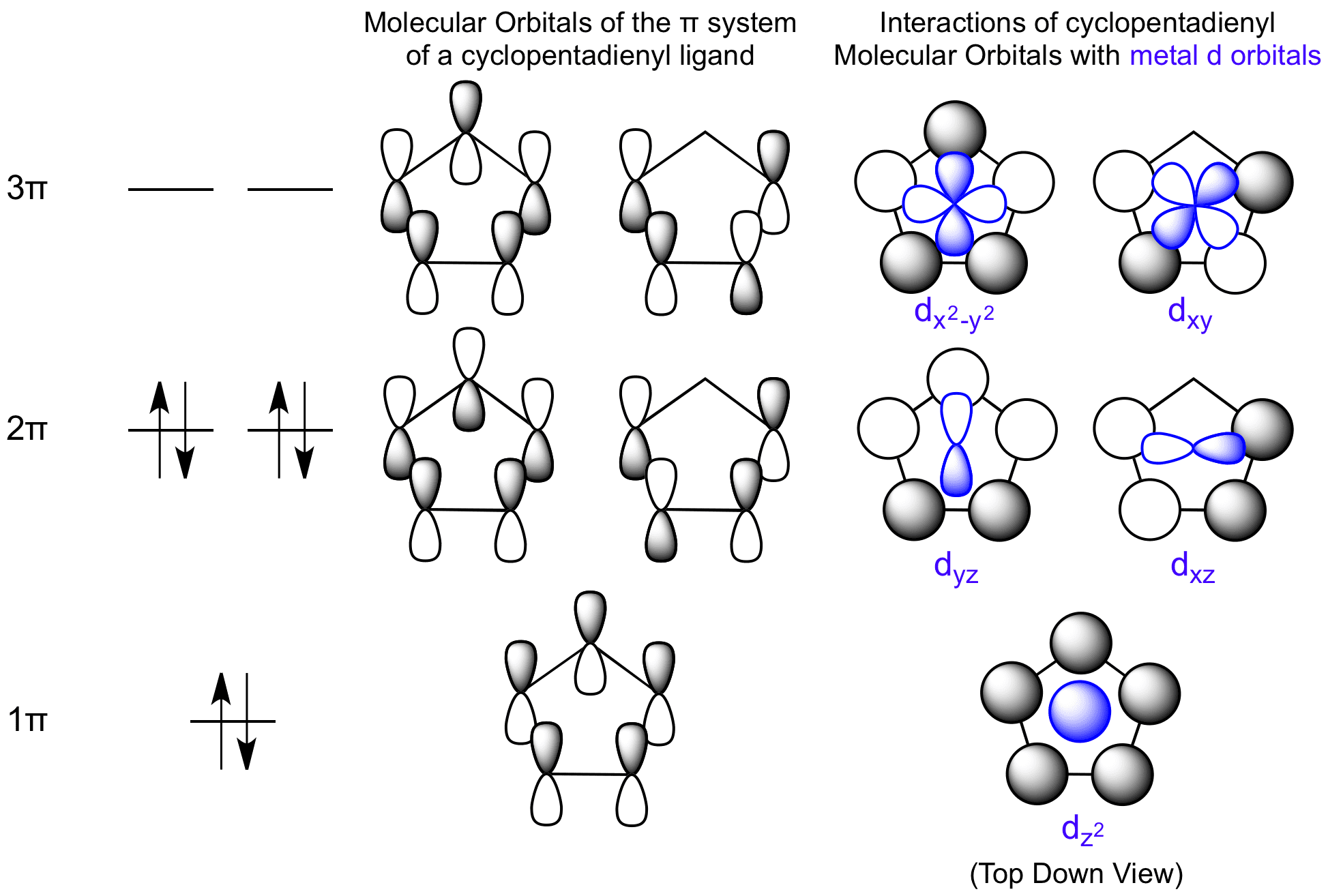
Energies of the pi molecular orbitals of cyclooctatetraene and the cycloheptatrienyl carbocation.Note that the electrons are not yet shown in the MOs in these diagrams. Figure 16-8 shows that the first 3 pairs of electrons are in three bonding molecular orbitals of cyclooctatetraene. Electrons 7 and 8, however, are located in two different nonbonding orbitals.
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
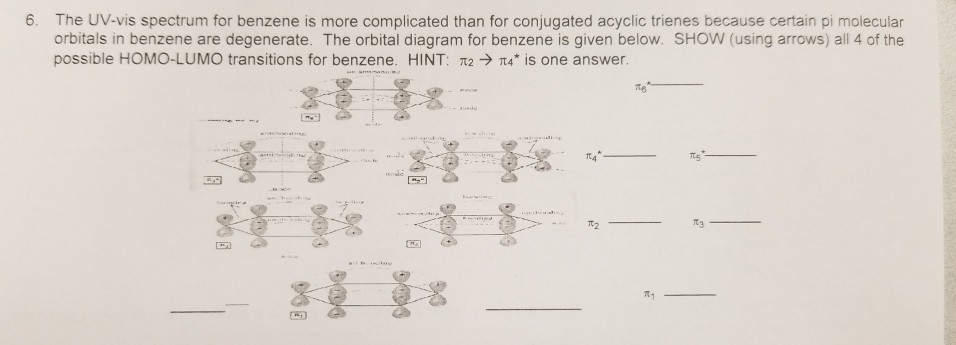
In pi 1 molecular orbital of 1,3,5-hexatriene there are 5 stabilizing bonding interactions where there are 6 stabilizing bonding interactions in the pi 1 of benzne. The sixth bonding interaction is made possible by benzene's p orbitals being in a ring.
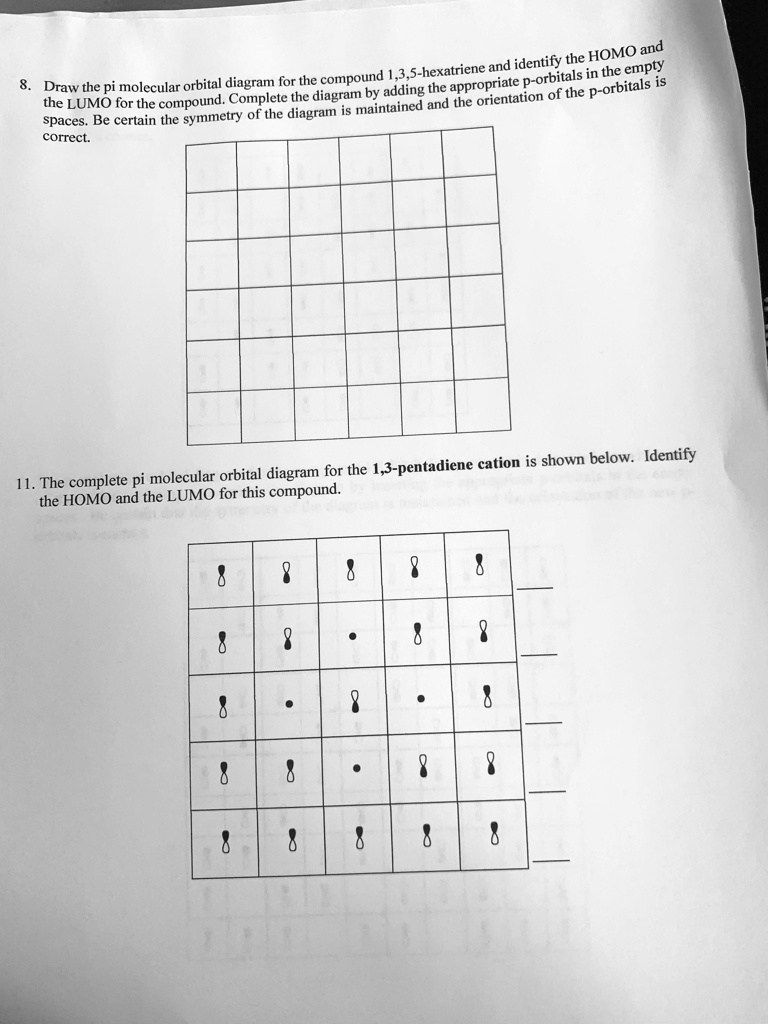
Solved Homo And 3 5 Hexatriene And Identibitahe In The Empty Draw The Pi Molecular Orbital Diagram For The Compound Appropriate P Orbitals Adding The P Orbitals The Lumo For The Compound Complete The Diagram By
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...

The Pi Molecular Orbitals Of Benzene Master Organic Chemistry In 2021 Chemistry Molecular Organic Chemistry
00:26 Reducible representation for pi group orbitals03:33 Reduction of reducible representation13:20 Effect of each symmetry operation on representativ...
The sum of these side-to-side interactions increases the electron probability in the region above and below a line connecting the nuclei, so it is a bonding molecular orbital that is called a pi (π) orbital (a bonding molecular orbital formed from the side-to-side interactions of two or more parallel np atomic orbitals).
Solved Draw The Pi Molecular Orbital Diagram For Each Of The Following And Label The Orbitals As Y1 W2 Etc Indicate Which Is The Homo And Lumo Course Hero
Molecular Orbital Diagram Atomic Orbital Pi Bond Aromaticity Png 2010x1140px Molecular Orbital Diagram Area Aromaticity Atomic










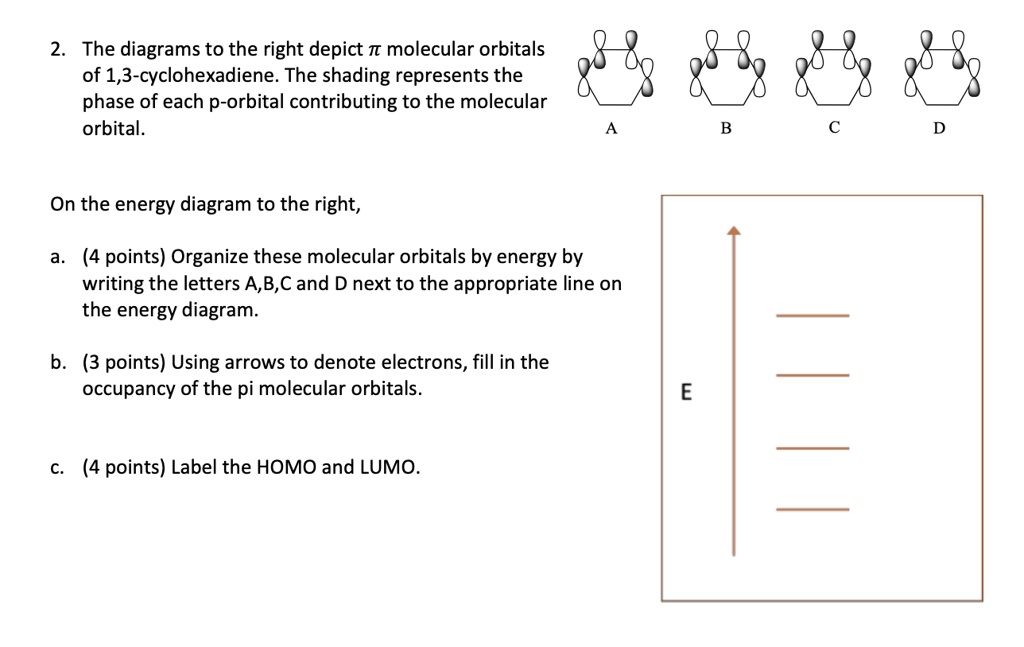
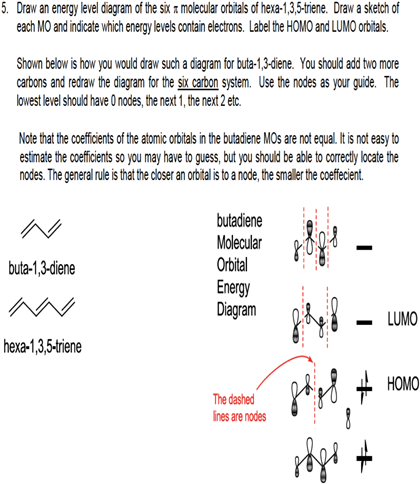
Comments
Post a Comment