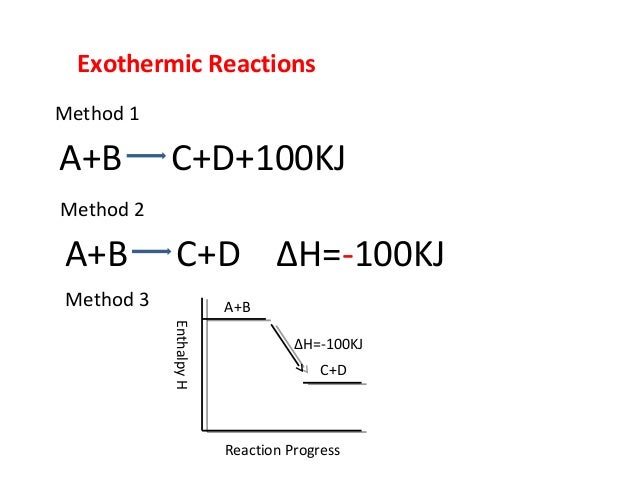
39 enthalpy diagram exothermic
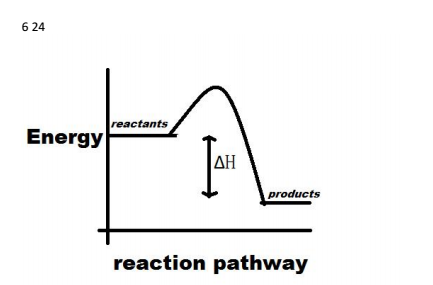
Energy level diagram - exothermic These show the relative energy levels (enthalpy) of reactants and products: H 2+ Cl 2 (products) Enthalpy exothermic 2HCl (reactants) Note that the specific reactants and products are shown for the reaction taking place. Reaction profile diagram Enthalpy diagram exothermic. Exothermic Standard Version - Exothermic Exothermic Reaction (3 of 3) - Stock Image - C002/8012 - Science Photo Library Exothermic Reactions - General Chemistry - Chemistry . Exothermic And Endothermic Processes Introduction To Chemistry ...
c) Draw a labeled enthalpy level diagram for an exothermic and endothermic reaction showing the activation energy, Ea and enthalpy change. [4] 9. (M05/S/2) In a neutralization reaction 50 cm 3 of a 0.50 moldm-3 solution of sodium hydroxide is mixed rapidly in a glass beaker with 50 cm 3 of a 0.050 moldm-3 solution of sulfuric acid.

Enthalpy diagram exothermic
Exothermic is when delta H is negative; energy is removed overall, so the ending energy is lower than the starting energy. In this endothermic diagram, the products have a higher enthalpy than the... 9. Draw an enthalpy diagram for a general exothermic reaction; label axis, reactants, products, and ∆H with its sign. 10. Draw an enthalpy diagram for a general endothermic reaction; label axis, reactants, products, and ∆H with its sign. 11. Write a balanced equation and draw an approximate enthalpy diagram for each of Enthalpy Change for Exothermic and Endothermic Reactions Chemistry Tutorial Key Concepts The enthalpy of a chemical system refers to the "heat content" of the system. 1 Enthalpy is given the symbol H Enthalpy change refers to the amount of heat released or absorbed when a chemical reaction occurs at constant pressure.
Enthalpy diagram exothermic. Hi everyone and welcome back to ASFC Chemistry!Click the little i to the top right hand corner of this video to be taken to some of our other A-level chemist... Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): combustion of one mole of methane vaporization of liquid alcohol freezing of liquid water formation of 1 mole of potassium chloride from its elements (heat is released) Learn this topic by watching Energy Diagram Concept Videos 5.1.4 - Deduce, from an enthalpy level diagram, the relative stabilities of reactants and products, and the sign of the enthalpy change for the reaction Enthalpy level diagrams, or energy profile diagrams, allow us to visualise what happens to the enthalpy of a reaction as it proceeds The total enthalpy of the reactant species is labelled H R Exothermic enthalpy diagram In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation. Enthalpy change is one of the most important concepts in chemistry. It does not matter what level of chemistry you are learning, your concepts of enthalpy change must be clear.
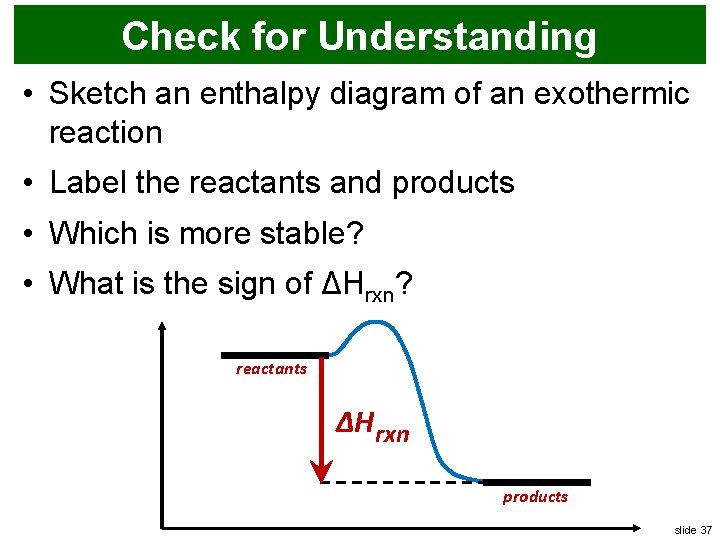
Enthalpy diagram for exothermic reaction. Products are more stable than reactants. Sets found in the same folder. Bond enthalpies. 5 terms. Tori-Kate. Measuring enthalpy changes. 5 terms. Tori-Kate. Other sets by this creator. Free energy change. 8 terms. Tori-Kate. Entropy. 7 terms. Tori-Kate. Enthalpies of solution. 11 terms. Tori-Kate. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... We can represent both enthalpy change and activation energy using a diagram that we call the energy profile diagram. Energy profile diagram for the combustion of hydrogen, which is typical of an exothermic reaction In the energy profile diagram showing the combustion of hydrogen, the energy level of the product is lower than that of the reactants. A negative Δ H \Delta H Δ H value means a reaction is exothermic, because the enthalpy of the products is less than the enthalpy of the reactants. This means that the reaction produced lower energy products from higher energy reactants, and the change in heat was released from the system to the surroundings.
Potential energy diagrams doc 27 kb drawing a potential energy diagram doc 31 kb spontaneous reaction worksheet doc 31 kb chemical reactions video the driving forces doc 26 kb entropy and enthalpy warm up doc 43 kb spontaneous entropy enthalpy and p e diagram questions docx 62 kb. Potential Energy Diagrams Worksheet Answers Keywords. Measuring the change in enthalpy allows us to determine whether a reaction was endothermic (absorbed heat, positive change in enthalpy) or exothermic (released heat, a negative change in enthalpy.) It is used to calculate the heat of reaction of a chemical process. Change in enthalpy is used to measure heat flow in calorimetry. A heat absorption reaction is endothermic. Its enthalpy will be positive, and its surroundings will cool down. This reaction (negative enthalpy, heat release) is exothermic. When the reaction happens, due to the gain in heat the device emits, the atmosphere may rise in temperature. In an exothermic reaction, the heat given out continues to provide the E A as the reaction continues. Endothermic reactions need to be heated (or an alternative energy supplied e.g. light) to keep providing the E A. We show enthalpy changes using an enthalpy profile diagram;, enthalpy on the y-axis
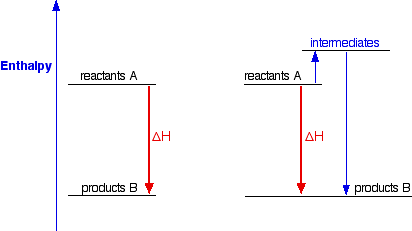
The overall enthalpy of the reaction is negative, i.e., it’s an exothermic reaction where energy is released in the form of heat. Depiction of an energy diagram In a chemical reaction, some bonds are broken and some bonds are formed.
Many chemical reactions release energy in the form of heat, light, or sound. These are exothermic reactions.Exothermic reactions may occur spontaneously and result in higher randomness or entropy (ΔS > 0) of the system. They are denoted by a negative heat flow (heat is lost to the surroundings) and decrease in enthalpy (ΔH < 0).
a) heat is a reactant b) heat is released c) the change in enthalpy is a negative number d) b&c Ос a . . d Ob Question 22 3.33 pts Consider the enthalpy diagram of an exothermic reaction. Where are the reactants in relation to the products on the H scale (y axis)?
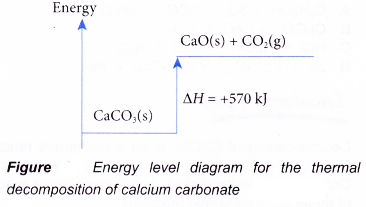
enthalpy of reactants = enthalpy of products + energy released H (N 2 (g) and H 2 (g)) = H (NH 3 (g)) + 92.4 kJ mol -1 We know the enthalpy change for the reaction: ΔH = -92.4 kJ mol -1 . (Remember the minus sign (-) tells us energy is released, energy is a product of the reaction, the reaction is exothermic.)
Nov 23, 2021 · Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
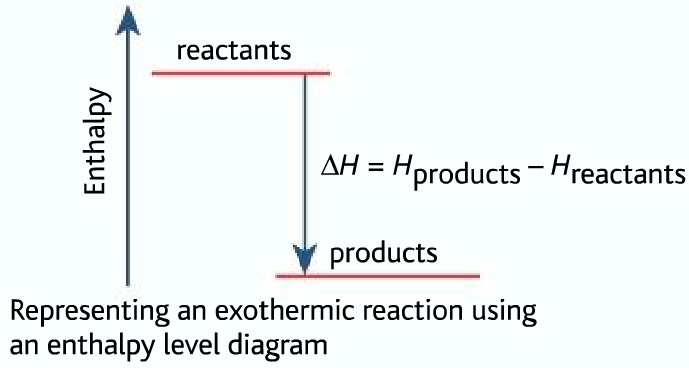
An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
In an exothermic reaction change in enthalpy ΔH will be negative. A chemical reaction always involves a change in energy. An energy level diagram shows whether a reaction is exothermic or endothermic. Hesss law and reaction enthalpy change. In endothermic reactions the energy of the reactants is lower than that of the products.
Given the reaction: A + B --> Ca) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for ...
Enthalpy change can be calculated from the potential energy diagram. Picture7 In National 5, you encountered the concept of Exothermic Reactions and ...
The enthalpy diagram for exothermic and endothermic reactions is shown below. Explanation : Endothermic reaction : It is defined as the chemical reaction in which the energy is absorbed from the surrounding. In the endothermic reaction, the energy of reactant are less than the energy of product.
Representing endothermic and exothermic processes using energy diagrams. AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
- Enthalpy, H, increase up the y-axis - The arrow in the enthalpy diagram points from reactants to products - The direction of the arrow in an enthalpy diagram indicates whether a reaction is exothermic or endothermic - In an endothermic reaction, the reactants will be at the bottom of the enthalpy diagram
Enthalpy Change for Exothermic and Endothermic Reactions Chemistry Tutorial Key Concepts The enthalpy of a chemical system refers to the "heat content" of the system. 1 Enthalpy is given the symbol H Enthalpy change refers to the amount of heat released or absorbed when a chemical reaction occurs at constant pressure.
9. Draw an enthalpy diagram for a general exothermic reaction; label axis, reactants, products, and ∆H with its sign. 10. Draw an enthalpy diagram for a general endothermic reaction; label axis, reactants, products, and ∆H with its sign. 11. Write a balanced equation and draw an approximate enthalpy diagram for each of
Exothermic is when delta H is negative; energy is removed overall, so the ending energy is lower than the starting energy. In this endothermic diagram, the products have a higher enthalpy than the...

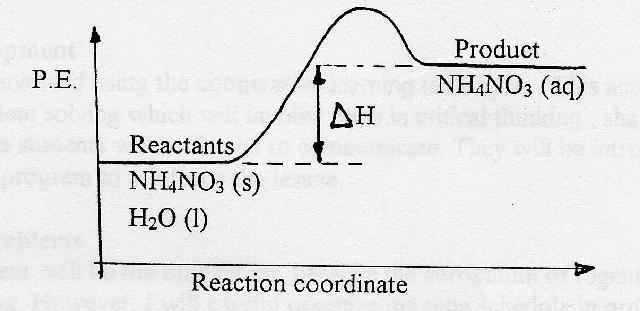
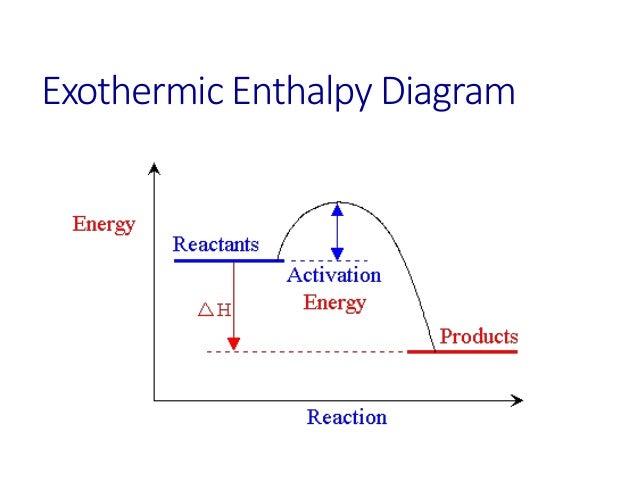
![5.3 Deduce, via enthalpy level diagrams the stabilitiy of reactants and products [SL IB Chemistry]](https://i.ytimg.com/vi/ijdhORfkOY0/maxresdefault.jpg)

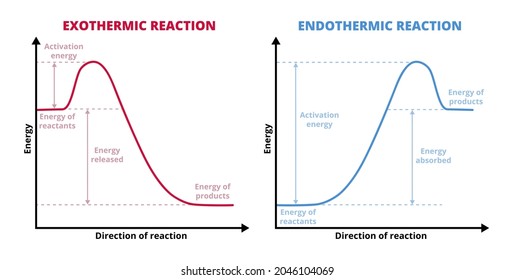
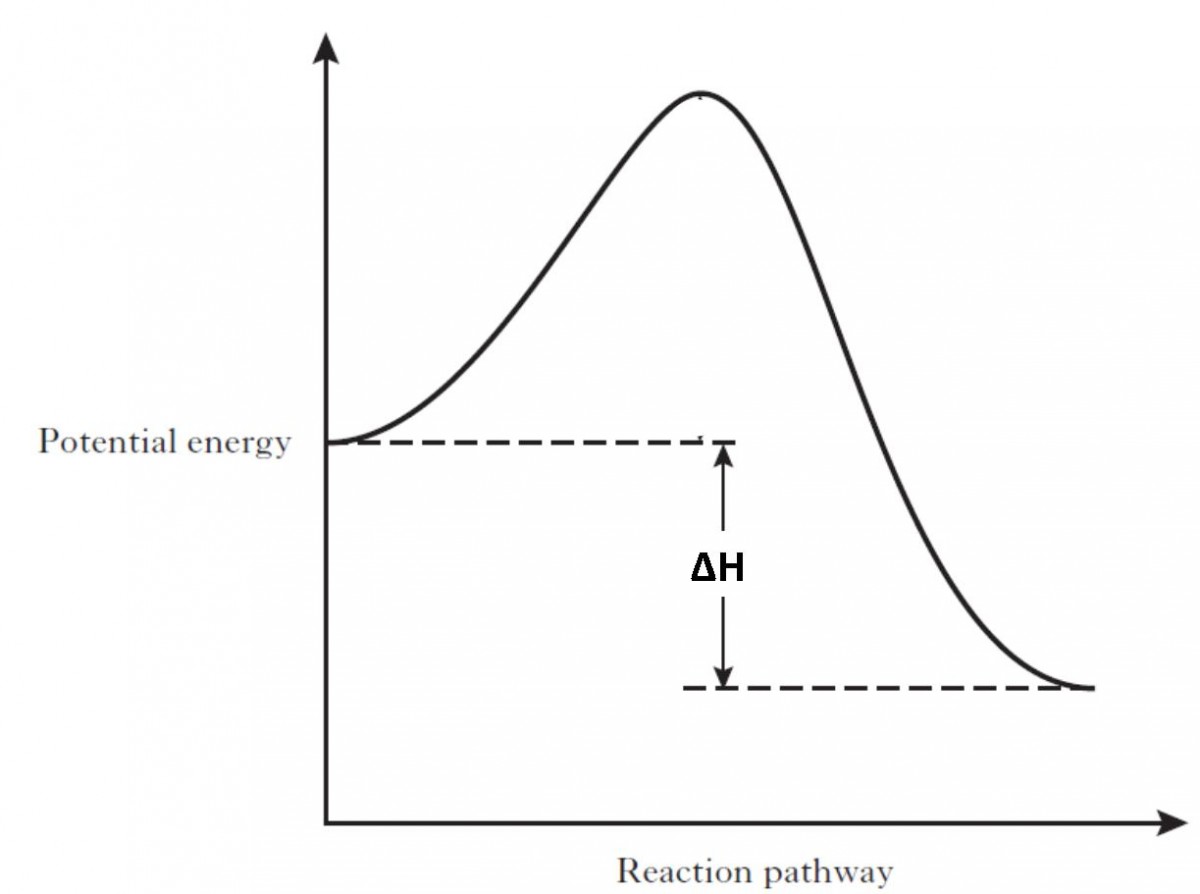




















Comments
Post a Comment