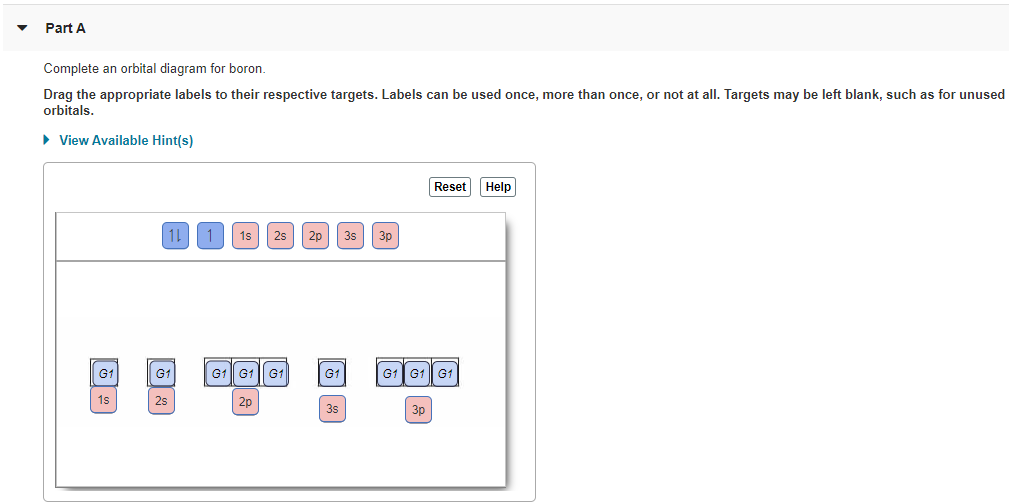
39 orbital diagram of boron
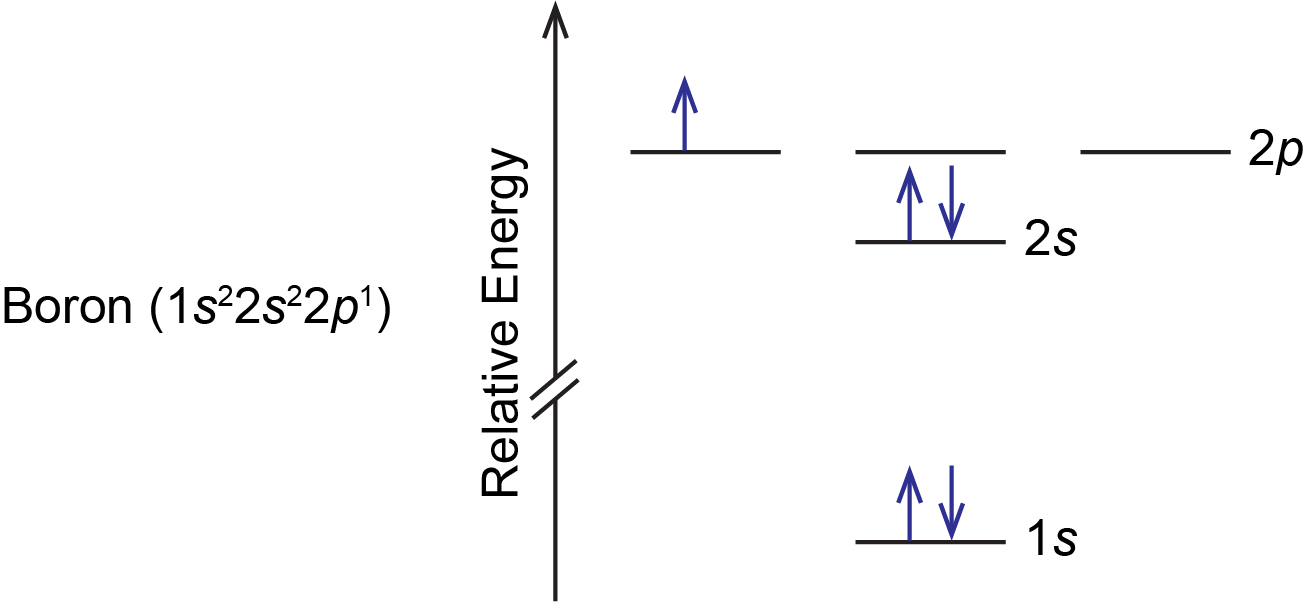
Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Draw an orbital diagram for scandium (Sc). From the orbital diagram, we can write the electron configuration in an abbreviated When we reach boron, with Z = 5 and five electrons, we must place the fifth . After filling the first five rows, we still have 80 − 54 = 26 more.Orbital Filling Diagrams. An orbital filling diagram is the more visual ...
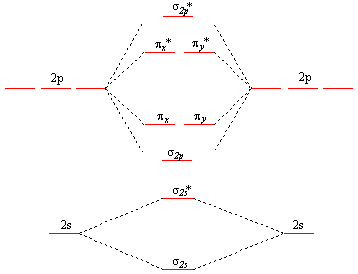
• MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.

Orbital diagram of boron
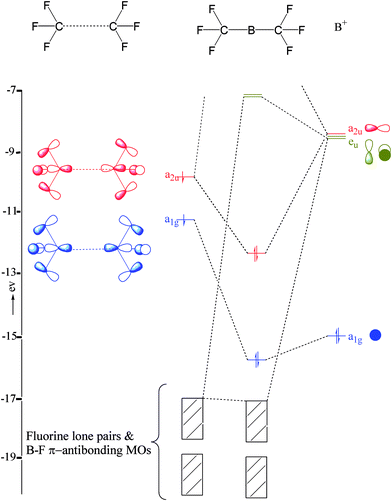
Show activity on this post. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2s for F considered non bonding. 2p (along the bond axis) for F has an ... The electron configuration of titanium and the orbital diagram is the main topic in this article. Also, valency and valence electrons of titanium, and compound formation, bond formation have been discussed. Hopefully, after reading this article you will know in detail about this. ... Boron is the 5th element in the periodic table and the first ... Orbital diagram. Boron electron configuration ← Electronic configurations of elements . B (Boron) is an element with position number 5 in the ... configuration of the Boron atom: 1s 2 2s 2 2p 1 Reduced electronic configuration B: [He] 2s 2 2p 1. Below is the electronic diagram of the Boron atom Distribution of electrons over energy levels in ...
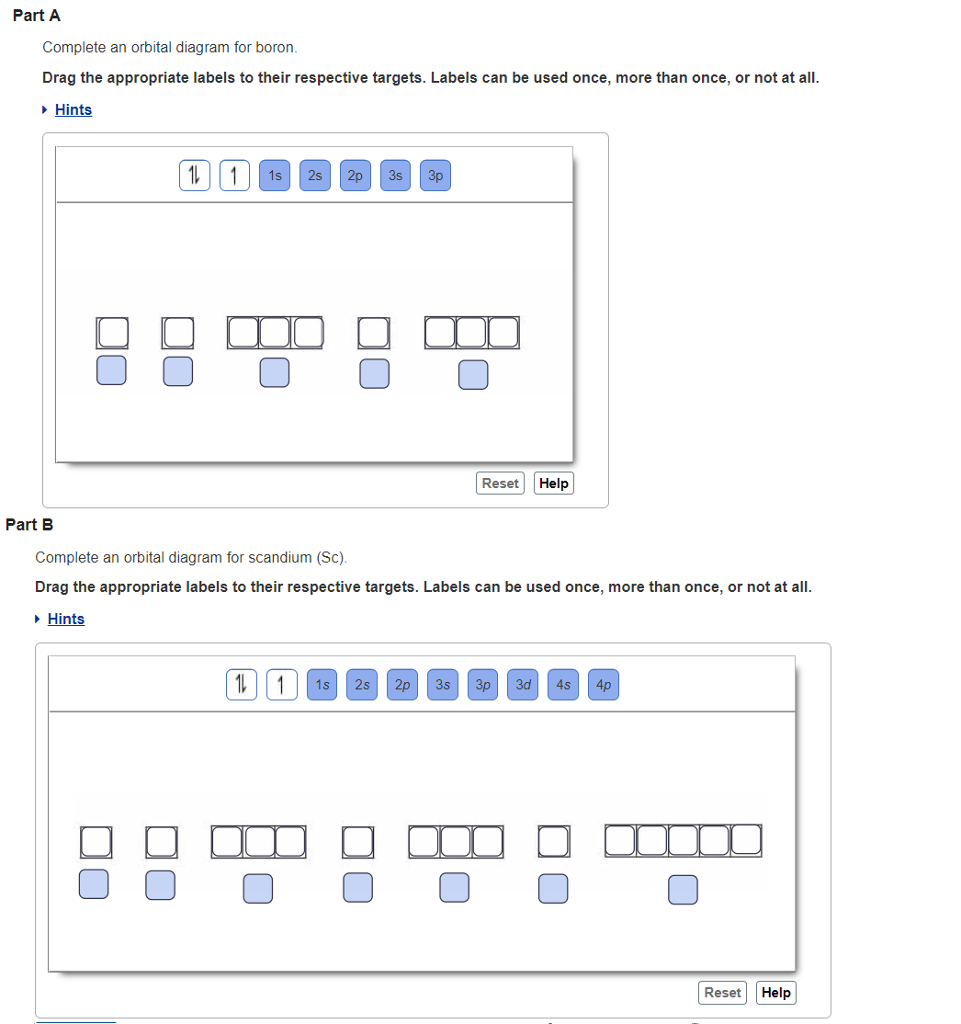
Orbital diagram of boron. Problem: Part A. Complete an orbital diagram for boron.Draw orbital diagrams, and use them to derive electron configurationsTo understand how to draw orbital diagrams, and how they are used to write electron configurations.The electron configuration of an element is the arrangment of its electrons in their atomic orbitals. Electron configurations can be used to predict most of the chemical ... 6. There is one p orbital on boron but there is no adjacent atom with another p orbital. Add it to the molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. Molecular Orbital Diagram of Boron Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.Wa... Boron has electronic configuration as , So, the excited state of will be , Here, Boron in excited state has one unpaired electron in 2p orbital rest 2 electrons are paired in the 2p orbital. This will make as a paramagnetic substance.
To write the orbital diagram for the Boron atom (B) first we need to write the electron configuration for just B. To do that we need to find the number of e... Boron (B) electron configuration with full orbital diagram Boron is the 5th element in the periodic table and the first element in group-13. The atomic number of boron is 5 and its symbol is 'B'. The standard atomic mass of boron is 10.806. The period of boron is 2 and it is a p-block element. (a) Starting with the orbital diagram of a boron atom, describe the steps needed to construct hybrid orbitals appropriate to describe the bonding in BF3. (b) What is the name given to the hybrid orbitals constructed in (a)? (c) On one origin, sketch the large lobes of the hy- brid orbitals constructed in part (a). (d) Are there any Fluorine SALCs and Boron AOs! Assume that fluorine 2s orbitals are not involved in the bonding and only consider the 2p orbitals. ! The 2p z orbital on each fluorine is perpendicular to the BF 3 plane and capable of forming out-of-plane pi interactions (ð z).! The 2p x orbital points toward the B atom and forms sigma interactions (ó)! The 2p y
B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3. In the formation of B 2 molecule, three valence electrons of each boron atom i.e. 6 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies. MO electronic configuration: This is the general MO diagram you need to fill with the valence electrons of BN Boron has 3 valence electrons, and nitrogen has 5 valence electrons, this makes 8 electrons. You have to start filling the orbitals from those with lowest energy to those with higher energy. So, 2 electrons on σ2s , two electrons on σ∗2s, two electrons on σ2p . in this problem were asked to start by sketching the orbital diagram of boron. And I'm only going to draw the second energy level. And boron has two electrons and the two s and one electron In the three peat. So the steps needed to construct the hybrid orbital's are one of these s electrons is promoted. So I've got one electron in each of three lobes. The electron configuration of niobium and the orbital diagram is the main topic in this article. Also, valency and valence electrons of niobium, compound formation, bond formation have been discussed. Hopefully, after reading this article you will know in detail about this. ... Boron is the 5th element in the periodic table and the first ...
An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ...
Molecular Orbital Diagram of Boron Nitride. #Molecular #Orbital #Diagram #Boron Nitride. All About Chemistry. 307 followers. Chemistry Classroom. Class 8. Free Website. Diagram. Teaching Chemistry. More information.... More like this. Organic Chemistry. Math Equations. Freund Reaction. All About Chemistry ...
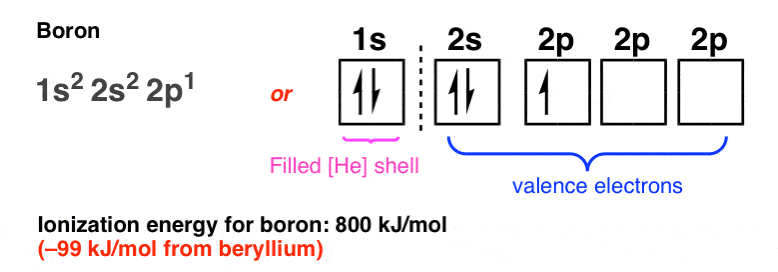
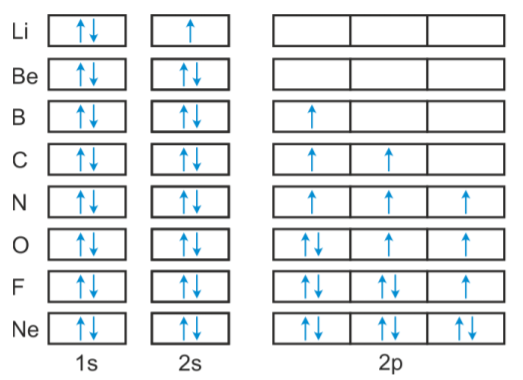
The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of. The next element is beryllium which has four electrons. The orbital diagram for beryllium is shown here.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Electron Configuration for Boron (B)Electron Configuration for Boron (B)
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s22s22p1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As).
In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. 1s2, 2s2, 2p1 Boron 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d1. Scandium.
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
Molecular orbital diagrams give us an idea about the mixing of orbitals in molecules. Let's look into the MO diagram of boron trichloride. The blue color refers to the atomic orbitals of boron, the red color refers to the atomic orbitals of chlorine and the molecular orbital of the molecule is indicated by the purple color.
Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically as an integer. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of. Question: Draw an orbital diagram for boron.
Orbital diagram. Boron electron configuration ← Electronic configurations of elements . B (Boron) is an element with position number 5 in the ... configuration of the Boron atom: 1s 2 2s 2 2p 1 Reduced electronic configuration B: [He] 2s 2 2p 1. Below is the electronic diagram of the Boron atom Distribution of electrons over energy levels in ...
The electron configuration of titanium and the orbital diagram is the main topic in this article. Also, valency and valence electrons of titanium, and compound formation, bond formation have been discussed. Hopefully, after reading this article you will know in detail about this. ... Boron is the 5th element in the periodic table and the first ...
Show activity on this post. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2s for F considered non bonding. 2p (along the bond axis) for F has an ...
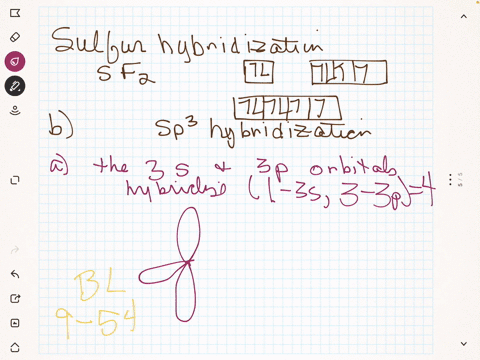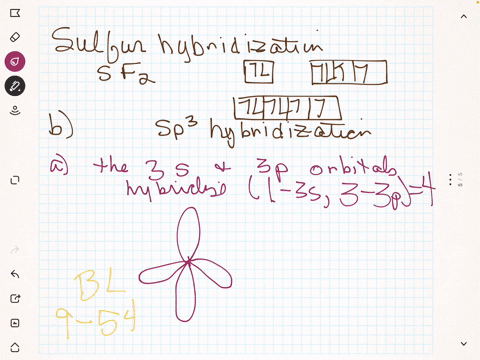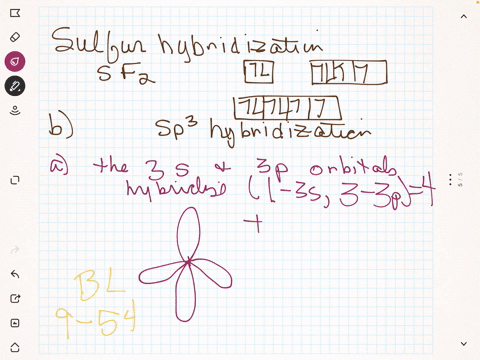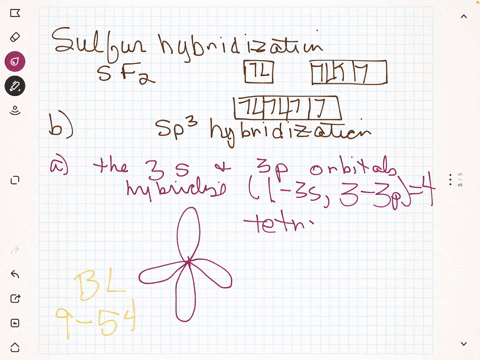





















Comments
Post a Comment