42 nickel copper phase diagram
Atomic percent nickel Phase diagram for Gold-Nickel showing complete solid solubility above about 800oC and below about 950oC. The miscibility gap at low temperatures can be understood with a regular solution model. Figure by MIT OCW. 800 700 600 500 400 300 200 100 0 10 20 30 40 50 60 70 80 90 100 Alpha Liquid Beta Atomic Percent Lithium DoITPoMS Micrograph Library Phase Diagram for Cu-Ni. Micrograph Library Browse the libraryAdvanced searchSystemsCompositionsTechniquesKeywordsPhase diagrams.
Not obvious in these plots but evident in the copper-nickel phase diagram is a tendency to immiscibility in the fcc phase at low temperatures. This contributes to a further transformation at 750 K, in which the amount of copper combined with sulphur as digenite sharply declines, whereas that of nickel and iron combined with sulphur in ...
Nickel copper phase diagram
This phase diagram is helpful in the analysis of 2618-type heat-resistant alloys and 339.0-type piston alloys that contain nickel, copper, and other alloying components (Tables 7.1 and 7.2). A good interpretation of a binary phase diagram that is easy to understand and interpret is the Cu-Ni system. This diagram has three different phase regions ... The preparation of Copper and Nickel nanoalloys is one of the key factors in determining the experimental phase diagrams. Various methods are available to alloy the nano particles. In another work by Irena Ban et al [ 5 ], Cu and Ni nanoparticles were alloyed using ball milling.
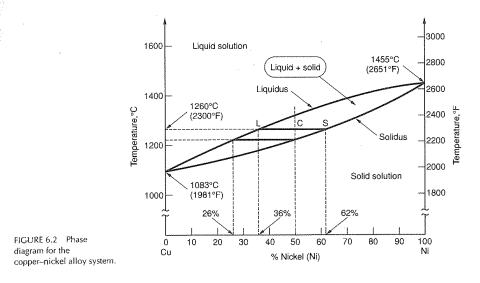
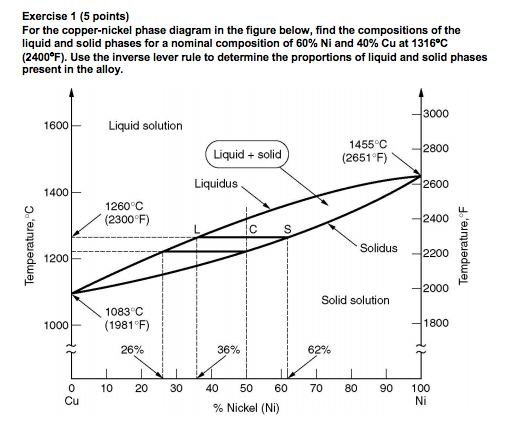
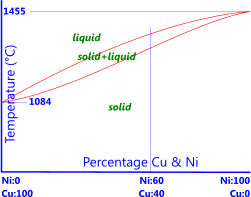
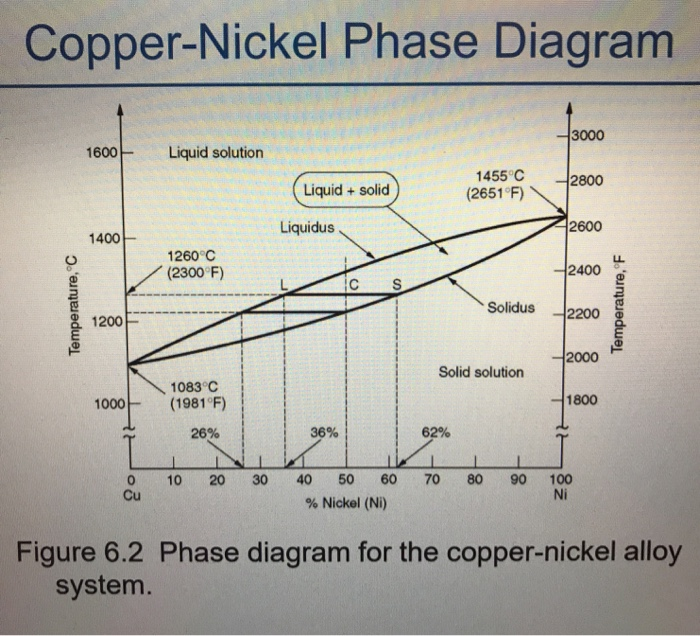
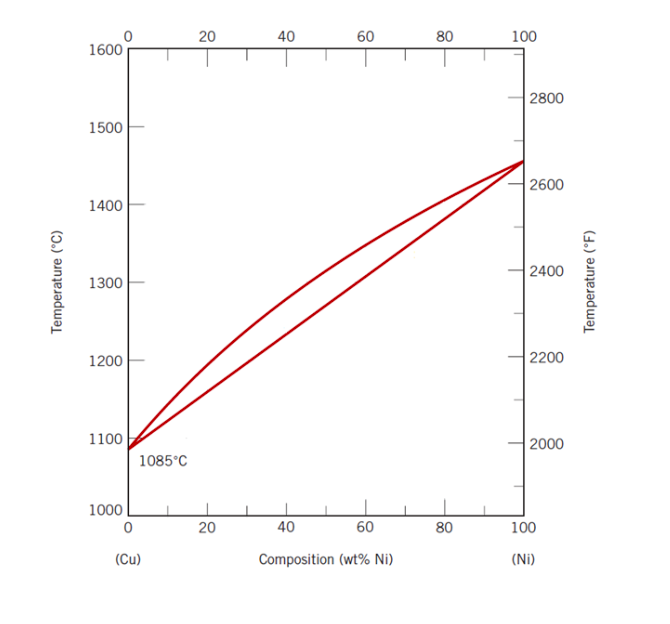
Nickel copper phase diagram. The binary phase diagram shown for the copper-nickel alloy indicates that these materials can form both liquid and solid solutions over the full range of composition from Cu to Ni. Above 1728 K, the melting point of pure Ni the alloys ar in the liquid phase. Between 1728 K and 1357 K (the melting point of Cu) the alloys can be either solid or liquid or exist as two phases in equilibrium. Phases and Phase Diagrams. Activity I: Phases and . Solid Solution Binary . Alloys. A glass of water has sugar slowly added to it until it saturates and a small amount of sugar is left at the bottom. A small amount of extra sugar is then added to the system and students are asked to predict what will happen to the sugar. Abstract. This article is a compilation of ternary alloy phase diagrams for which copper (Cu) is the first-named element in the ternary system. The other elements are Fe, Mn, Ni, Pb, S, Sb, Si, Sn, Ti and Zn. The diagrams are presented with element compositions in weight percent. The article includes 42 phase diagrams (liquidus projection ... Introduction to the Cu-Ni phase diagram relating to the use of the Lever Rule for determining composition and phase weight percentages.
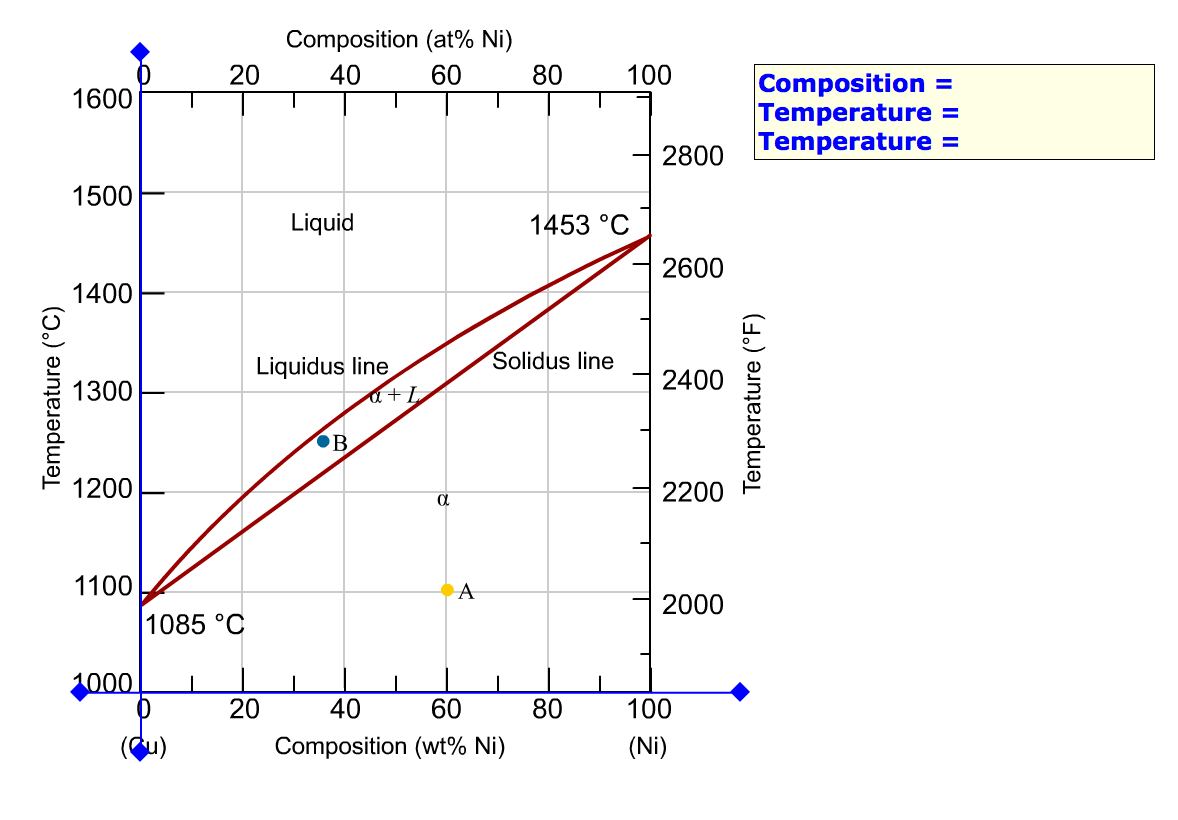
As-cast and annealed samples were studied metallographically; melting-point and x-ray studies also aided in defining phase relationships. The melting point of yttnium metal was sharply reduced with small additions of either iron, nickel, or copper. Less than 1 wt.% iron, nickel, or copper was soluble in solid yttnium. Shown is a partial binary phase diagram for the Copper-Nickel system with the alloy Cu-35 wt% Ni at the vertical line. At the point, a, the alloy is a liquid. Upon cooling it passes through the two-phase ( a + L) zone and then solidifies as a single phase substitutional alloy. Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash Journal of Phase Equilibria - 13Ces: P. de Cesaris, "The Ternary Alloys of Nickel, Copper and Silver,"Gass. Chim. Ital., 43(2), 365-379 (1913) in Italian.(Equi ...
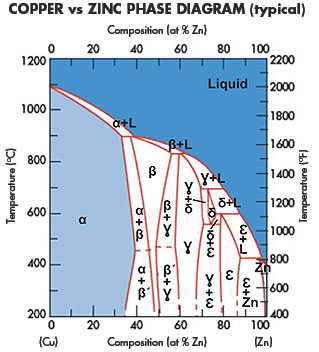
Consider the binary phase diagram for copper-nickel system, as shown. The abscissa ranges from 0 wt% Ni (100 wt% Cu) to 100 wt% Ni (0 wt% Cu). Three different phase regions (or fields) appear in the diagram: anα field, a liquid (L) field, and a two-phase α+ L field. The liquid L is a homogeneous liquid solution K.P. Gupta, The Cu-Fe-Ni (Copper-Iron-Nickel) System, Phase Diagrams of Ternary Nickel Alloys. Part 1 , 1990, Indian Institute of Metals, Calcutta, p 290-315 1991Swa The copper - zinc phase diagram is a bit simpler than the copper - tin phase diagram but still complex enough. There are all kinds of brass' but typically we are at the copper-rich side. Of course, if we want to look at all copper alloys, we would need a bunch of more binary phase diagrams, in particular for the elements arsenic (As), antimony, (Sb), silver (Ag), and lead (Pb) since these are ... EN380 Homework #6 Solution 1.The binary (two-component) phase diagram for copper-nickel alloys is shown below. A Cu - Ni alloy contains 30 wt. % Cu at a temperature of 1350 C. Find the following: (a)melting point for pure copper.1084 C (b)melting point for pure nickel.1455 C (c)wt. % Niin the (entire) binary alloy.70% Ni
the tie-line intersection with the β + L−β phase boundary occurs at 97.5 wt% Sn, which is the composition of the β phase. Thus, the phase compositions are as follows: Cβ = 97.5 wt% Sn-2.5 wt% Pb C L = 74 wt% Sn-26 wt% Pb 9.11 A copper-nickel alloy of composition 70 wt% Ni-30 wt% Cu is slowly heated from a temperature of 1300°C (2370°F).
The Ni-Cu alloy phase diagram. The initial condition of the ...
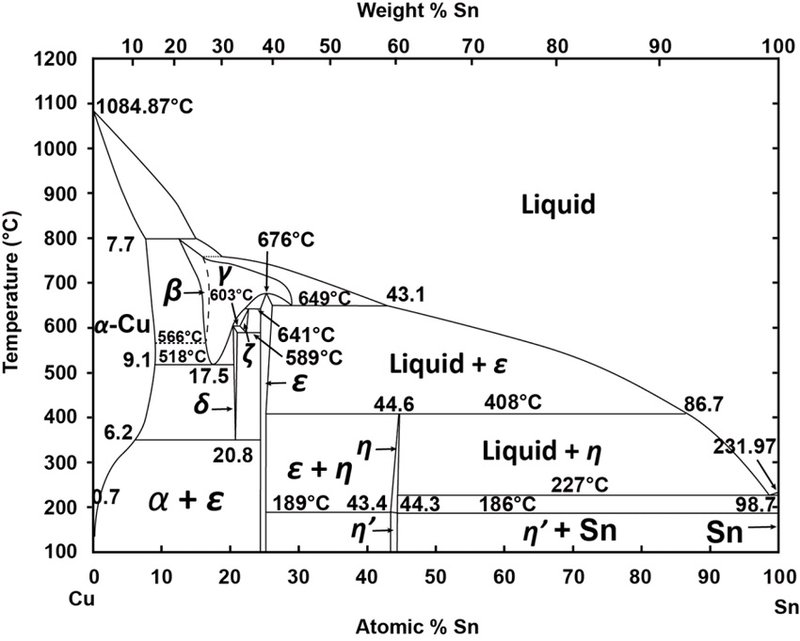
This representation is called a phase diagram. The phase diagrams of some binary systems relevant to bronze show the behavior of alloying elements that typically results in one of the three cases mentioned previously. The copper-tin equilibrium phase diagram (Figure 3) illustrates Cases (1) and (2).
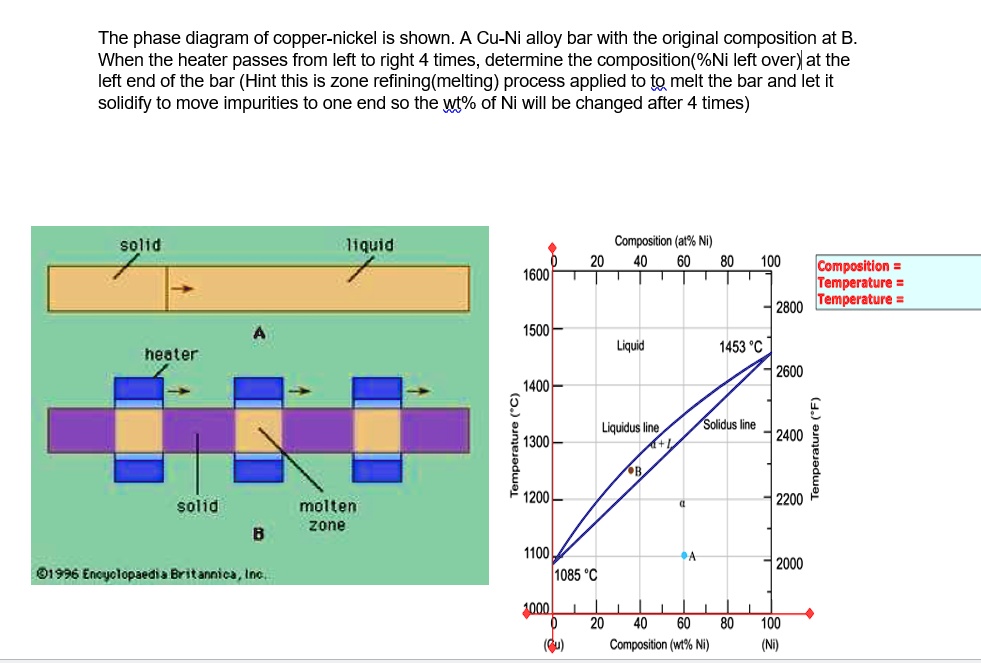
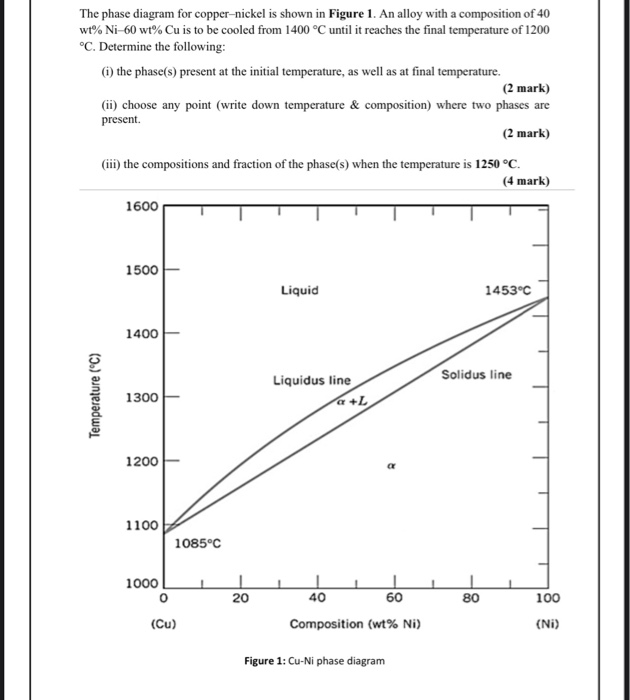
Question. : Problems Engineering Metals HomeWork #1 Problem 1 For the copper-nickel phase diagram below, find the compositions of the liquid and solid phases for a nominal composition of 65% Cu and 35% Ni at1200 °C and determine the proportion amount of liquid and solid phases present in the alloy. 3000 1600 Liquid solution 1455 € (2651F ...
The age-hardenable copper-nickel-silicon alloys with 1.0 to 4.5% Ni and 0.2 to 0.6% Be are not dealt with here. In European standards, these alloys are assigned to ‘low-alloyed copper alloys’ (see R 13388 and relevant product standards). Figure 1. (DKI A 4049) Cu-Ni equilibrium diagram
A copper-nickel alloy of composition 35 wt% Ni -65 wt% Cu is slowly cooled from 1300 oC (a)At what temperature does the first solid phase form? ... Example - Gibbs phase rule in eutectic diagram In the double phase regions: N = 1 (the diagram is plotted for fixed pressure, 1 atm) C = 2 P = 2 F = N + C -P F = 1
the thermal equilibrium diagram for the alloy of Copper and Nickel. In order to find what temperature 60% copper solidifies at we simply draw a vertical line from 60% copper until it hits the solidus line and at this is the point where 60% Copper has fully solidified. 0 100 10 90 20 80 30 70 40 60 50 50 60 40 70 30 80 20 90 10 100 0 900 1000 ...
57 Phase Diagram of the Nickel-Bismuth System 58 Phase Diagram of the Copper-Zinc System 59 Phase Diagram of the Copper-Aluminum System 60 Phase Diagram of the Copper-Germanium System 61 Phase Diagram of the Copper-Antimony System 62 Phase Diagram of the Copper-Tin System 63 Additional IV Curves of Solar Cells With Copper Contacts
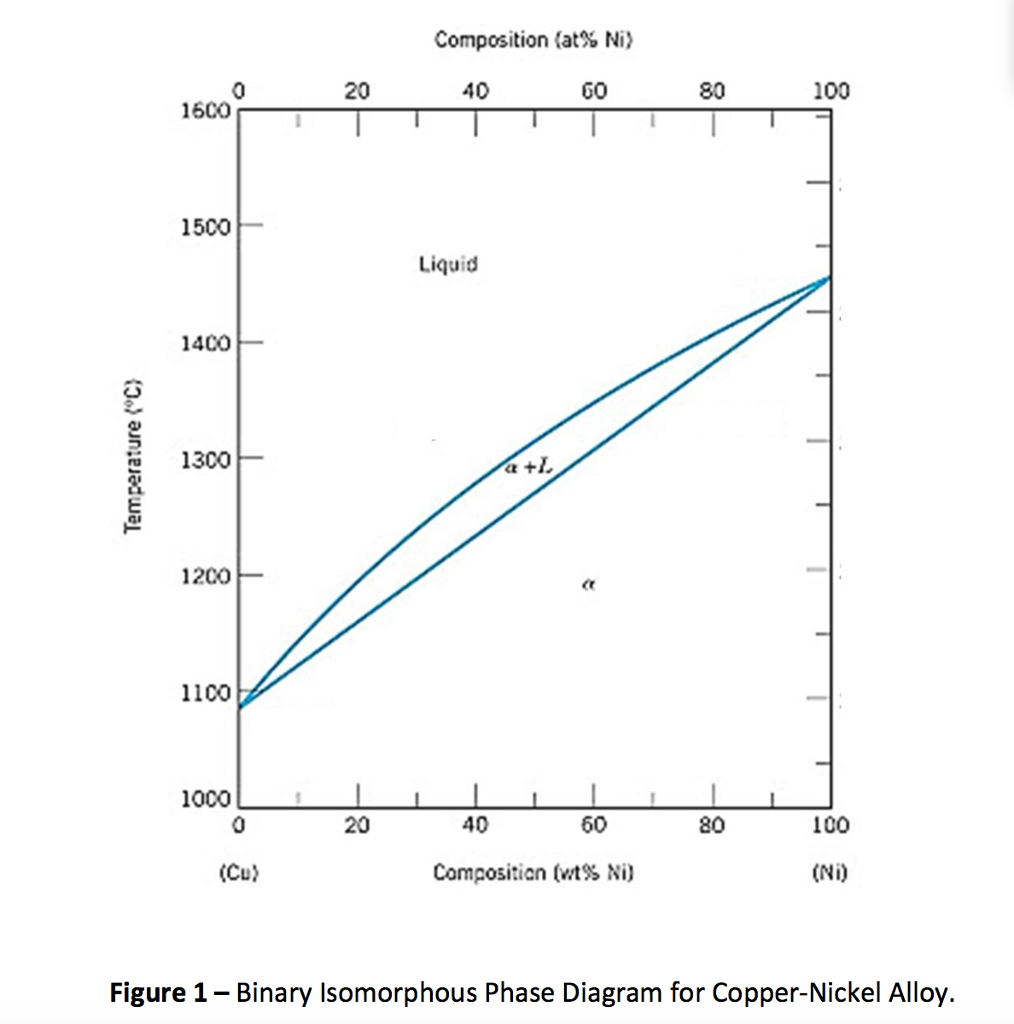
A good interpretation of a binary phase diagram that is easy to understand and interpret is the Cu-Ni system. This diagram has three different phase regions, the alpha region, the liquid region, and the alpha + liquid region, which are defined by specific compositions and temperatures as illustrated in figure 1.
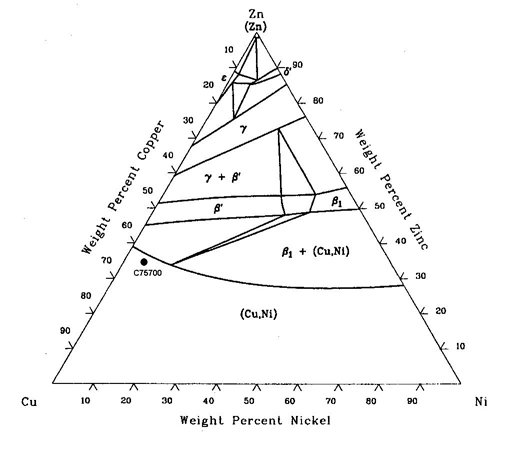
In 1751, A.F. Cronstedt succeeded in isolating nickel. However, Cu-Ni alloys were in existence much earlier, mostly prepared by processing ores. Today, Cu-Ni alloys have gained a variety of interesting applications because of their specific characteristics . Copper and nickel are adjacent to one another in the periodic system of elements, with atomic numbers 29 and 28 and atomic weights 63.54 and 68.71.The two elements are closely related and are completely miscible in both the liquid and solid state. Cu-Ni alloys crystallise over the whole concentration range in a face-centred cubic lattice. The lattice spacing of the face-centre cubic solid solution varies almost linearly with atomic concentration between the values for copper (3.6153 . 10-8cm) and that for nickel (3.5238 . 10-8cm). Cu-Ni alloys are alloys of copper (base metal with the largest individual content) and nickel with or without other elements, whereby the zinc content may not be more than 1%. When other elements are p...
The nickel-copper phase diagram is given in Figure 1 (2). Besides pure nickel and copper, important alloys in the system are nickel-30% copper (Monel) and copper-30% nickel (Cupro-Nickel). Monel is useful in applications where contact is made with corrosive environments, e.g., acids and seawater.
This video explains binary phase diagrams, specifically the Cu-Ni System. For further studies: visit https://www.doitpoms.ac.uk/tlplib/phase-diagrams/printal...
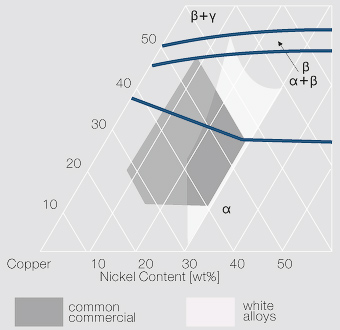
at the left of the diagram where the alloy is in solid solution (phase ), a face centred cubic structure like copper. For the typical alloy CuBe1.9 (1.8 to 2% of beryllium) the alloy is in the phase between 720°C and 860°C. For industrial purposes the solution heat treatment is done between 750° and 800°C. By rapidly cooling the
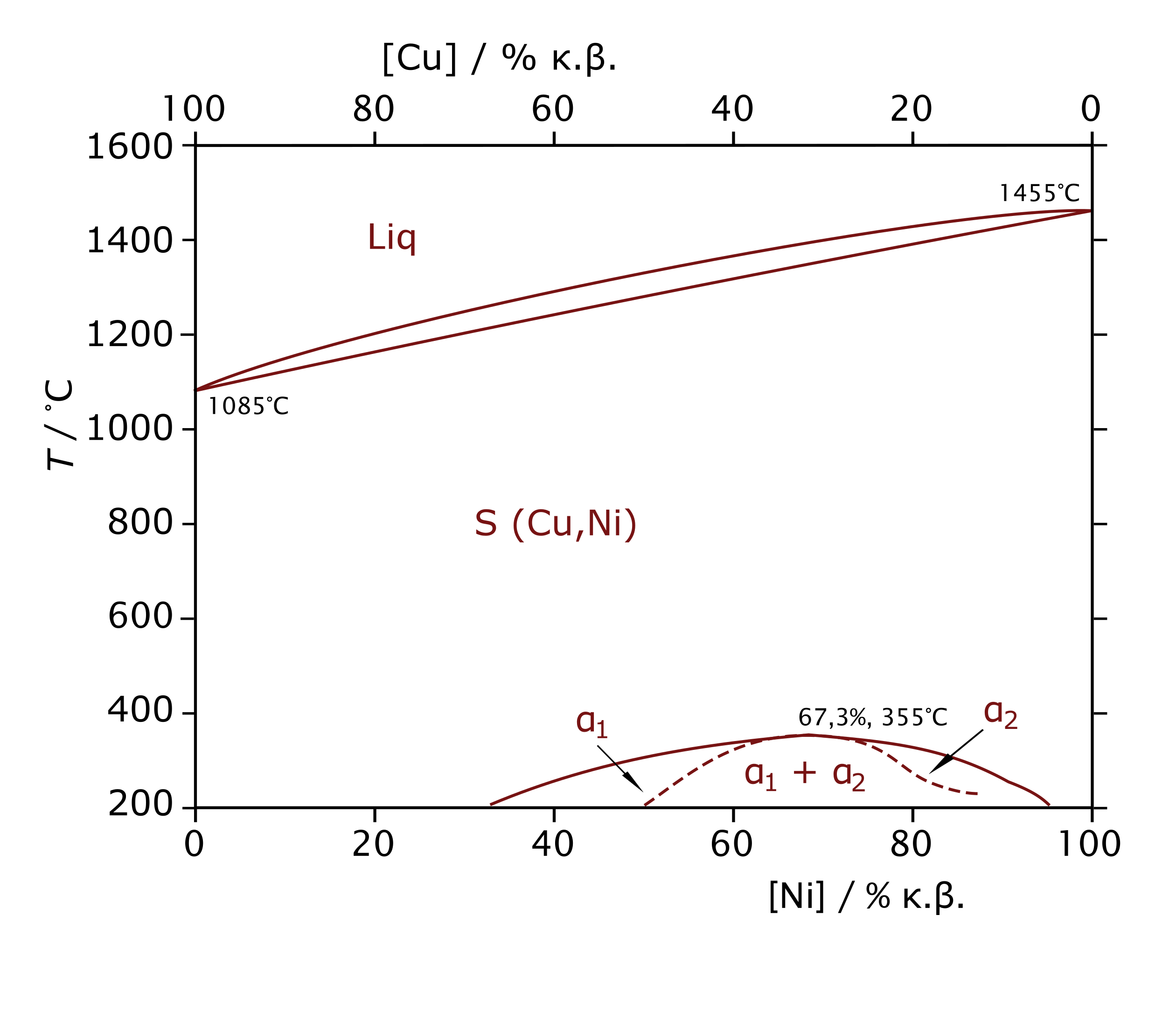
Copper-nickel phase diagram including the miscibility gap of the (Cu, Ni) phase: the dashed line denotes the boundary of magnetic transformation The immiscibility of the (Cu, Ni) phase is a ...
The phase diagram for Cu-Ni is shown in Figure 1203.01.02, where the diagram follows the normal presentation, with the ordinate as temperature and the abscissa is composition, plotted in this case as wt% Ni (Note: an abscissa in atomic % is an alternative presentation, but we will use weight % in this lecture).
The preparation of Copper and Nickel nanoalloys is one of the key factors in determining the experimental phase diagrams. Various methods are available to alloy the nano particles. In another work by Irena Ban et al [ 5 ], Cu and Ni nanoparticles were alloyed using ball milling.
A good interpretation of a binary phase diagram that is easy to understand and interpret is the Cu-Ni system. This diagram has three different phase regions ...
This phase diagram is helpful in the analysis of 2618-type heat-resistant alloys and 339.0-type piston alloys that contain nickel, copper, and other alloying components (Tables 7.1 and 7.2).



![Sn-Cu-Ni phase diagram [7]. | Download Scientific Diagram](https://www.researchgate.net/publication/317967778/figure/fig3/AS:510292191072256@1498674547588/Sn-Cu-Ni-phase-diagram-7.png)





![Cu-Ni phase diagram [2]. | Download Scientific Diagram](https://www.researchgate.net/profile/Andras-Kovacs-5/publication/321224714/figure/fig1/AS:631621728337962@1527601763764/Cu-Ni-phase-diagram-2.png)
![1 Phase diagram of copper nickel system [88]. | Download ...](https://www.researchgate.net/profile/Gandham-Phanikumar/publication/307963440/figure/fig14/AS:404634498813953@1473483790439/Phase-diagram-of-copper-nickel-system-88.png)




Comments
Post a Comment