43 lewis diagram for nitrogen
The Lewis structure is the foremost step to begin studying the physical and chemical properties of any molecule. For nitrogen trichloride, it is essential to study the Lewis structures of the participating atoms before drawing the one for the molecule. The atomic number of nitrogen and chlorine are 7 and 17. It makes their electronic configuration: N2 Lewis Structure Setup. It’s easiest to think in terms of dots to make the N 2 Lewis structure. Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom of the N atoms in the below diagram. There is also a pair of dots, representing two more electrons, that won’t bond, on top of each N.
A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ...

Lewis diagram for nitrogen
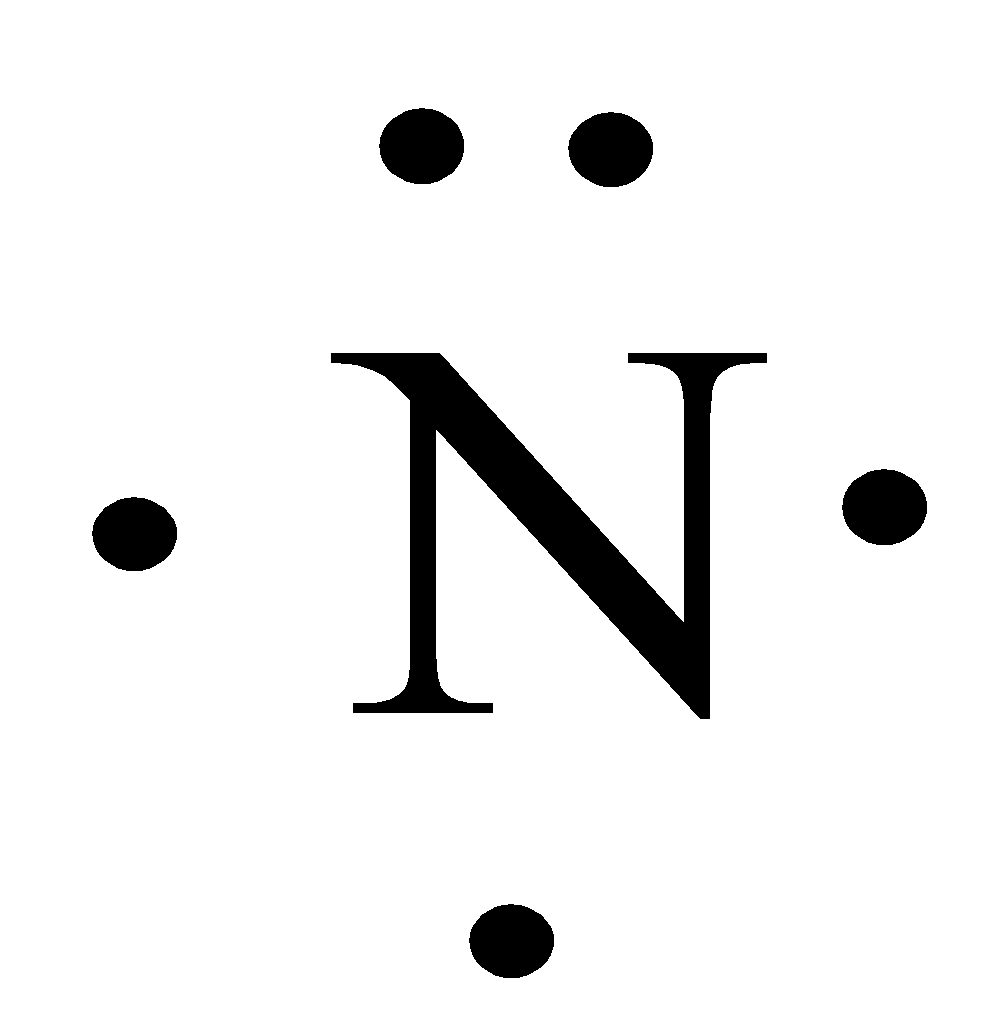
Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). Click to see full answer Thereof, what are electron dot diagrams used for? What is the Lewis structure for N2H4? N2H4 is straightforward with no double or triple bonds. In the N2H4 Lewis structure the two Nitrogen (N) atoms go in the center (Hydrogen always goes on the outside). Hydrogen (H) only needs two valence electrons to have a full outer shell. In the Lewis structure for N2H4 there are a total of 14 valence ... Drawing the Lewis Structure for N 2 ( Dinitogen or Nitrogen Gas) Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2 ). It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.
Lewis diagram for nitrogen. beryllium and nitrogen lewis dot structure. Lewis Structures are important to learn because they help us predict: the shape of a molecule. Step 1: Determine the total number of electrons available for bonding. Nitrogen (IV) oxide (NO 2) is a well-known example. Electron dots are typically arranged in four pairs located on the four "sides" of ... NH2OH lewis structure has two N-H bonds, one N-O bond, and one O-H bond. The nitrogen is the central atom and there is one lone pair on it. The lewis structure of NH2OH has a total of 3 lone pairs and 4 bond pairs. NH2OH lewis structure is drawn with the same procedure as the NH2Cl lewis structure. What is the Lewis dot structure for nitrogen gas? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. In the Lewis structure for NO2 the Nitrogen atom is the least electronegative atom and goes at the center of the structure. For the NO2 Lewis structure, calculate the total number of valence electrons for the NO2 molecule. After determining how many valence electrons there are in NO2, place them around the central atom to complete the octets.
Lewis Structure of N2H4 Lewis dot diagram or electron dot structure is the pictorial representation of the molecular formula of a compound along with its electrons that are represented as dots. These structures are named after American chemist Gilbert Newton Lewis who introduced them in 1916. Nitrogen (N) has 5 balance electron & Oxygen (O) has 6 balance electron. Now, the overall charge of NO2+ ion has +charge (NO2+). so, it reduce number of electron by (-1). Now, the total balance electron of NO2+ ion is, N=5, O2 = 6×2 = 12 and +charge = -1 So, 5+12-1 = 16, the balance electron of NO2+ ion is 16. For "dinitrogen gas", :N-=N:. For atomic nitrogen, Z=7. There are thus 7 electrons to distribute per nitrogen atom, 2 of which are inner core, and these may be ignored. So we distribute 5 electrons around EACH nitrogen centre and come up with a triple bond. This Lewis structure reflects the shortness and strength of the bond in the dinitrogen molecule, which are experimental findings. NO2 involves an sp2 type of hybridization. The most simple way to determine the hybridization of NO2 is by drawing the Lewis structure and counting the number of bonds and lone electron pairs around the nitrogen atom. You will find that in nitrogen dioxide there are 2 sigma bonds and 1 lone electron pair.
NO2 Lewis Dot Structure. Whenever something burns in the air, Nitrogen oxides will be formed. The reason for this is that the air we breathe mainly consists of Nitrogen (78%) and Oxygen (21%), and these combine in the presence of energy (from burning materials) in the environment. Apr 11, 2016 — The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration of ... Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. In our case, nitrogen dioxide is composed of 1 nitrogen atom and two oxygen atoms. Nitrogen is a group 15 element and so has 5 valence electrons, while group 16 oxygen has 6 valence electrons. There are two oxygen atoms, so the total amount of valence electrons in our diagram is: 5 (1) + 6 (2) = 17 electrons
The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. what is the first step in creating a Lewis structure for a molecule? How to Draw a Lewis Structure. Step 1: Find the Total Number of Valence Electrons. Step 2: Find the Number of Electrons Needed to Make the Atoms "Happy".
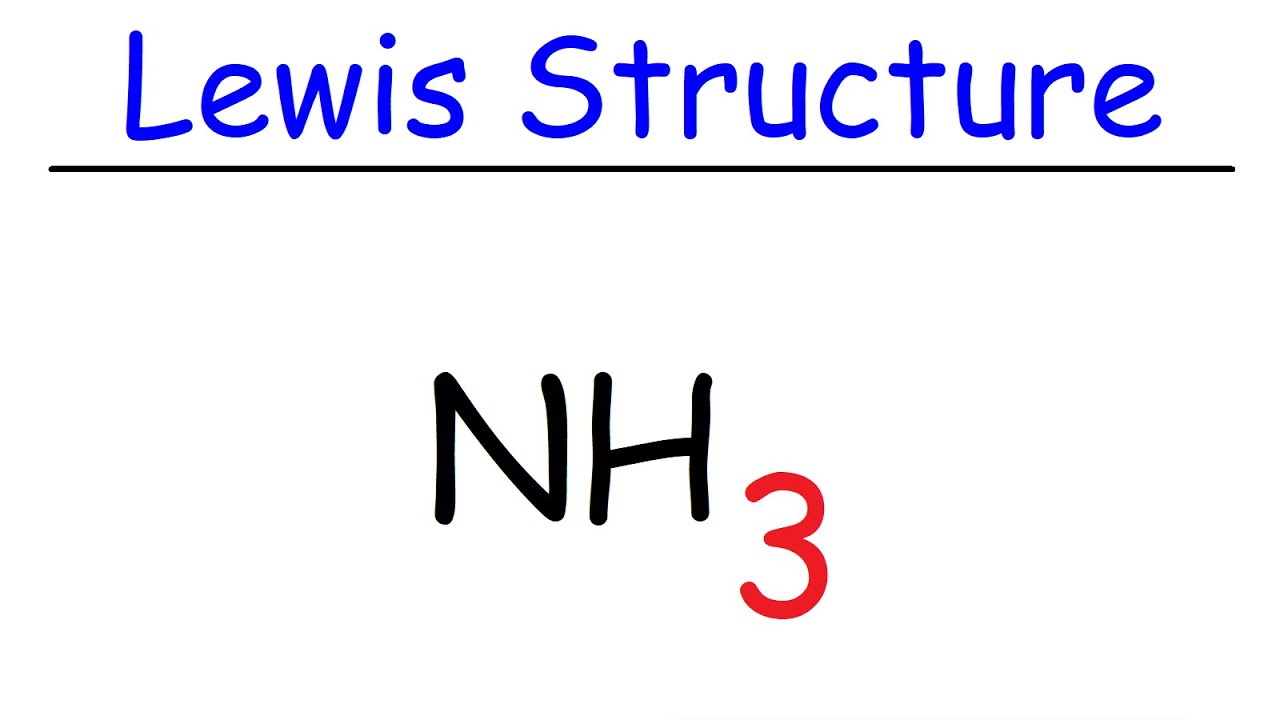
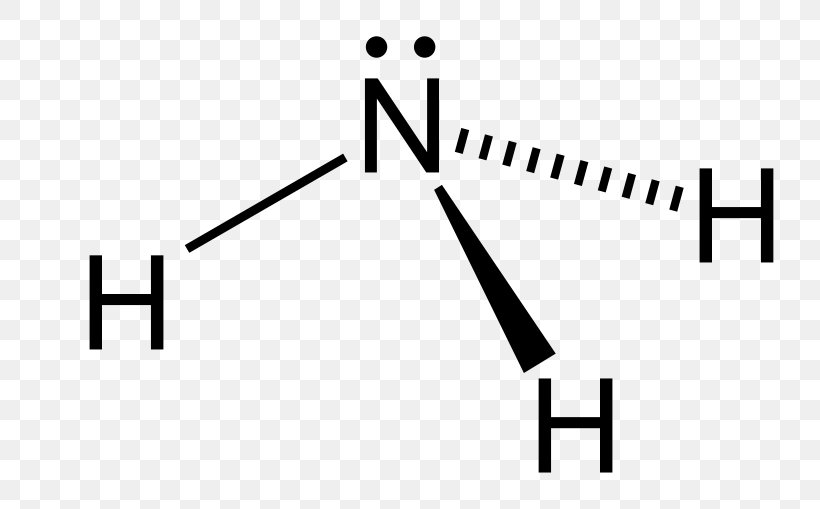
In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of ammonia? Out of which 3 electrons of nitrogen form a covalent bond with hydrogen ...
Jan 12, 2021 · What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.
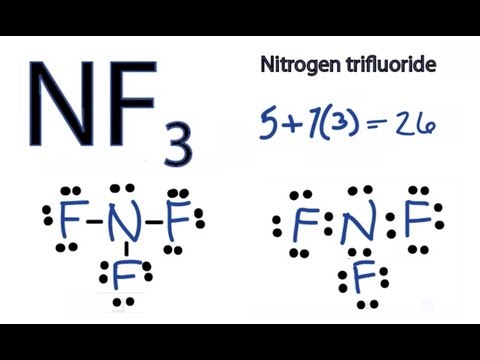
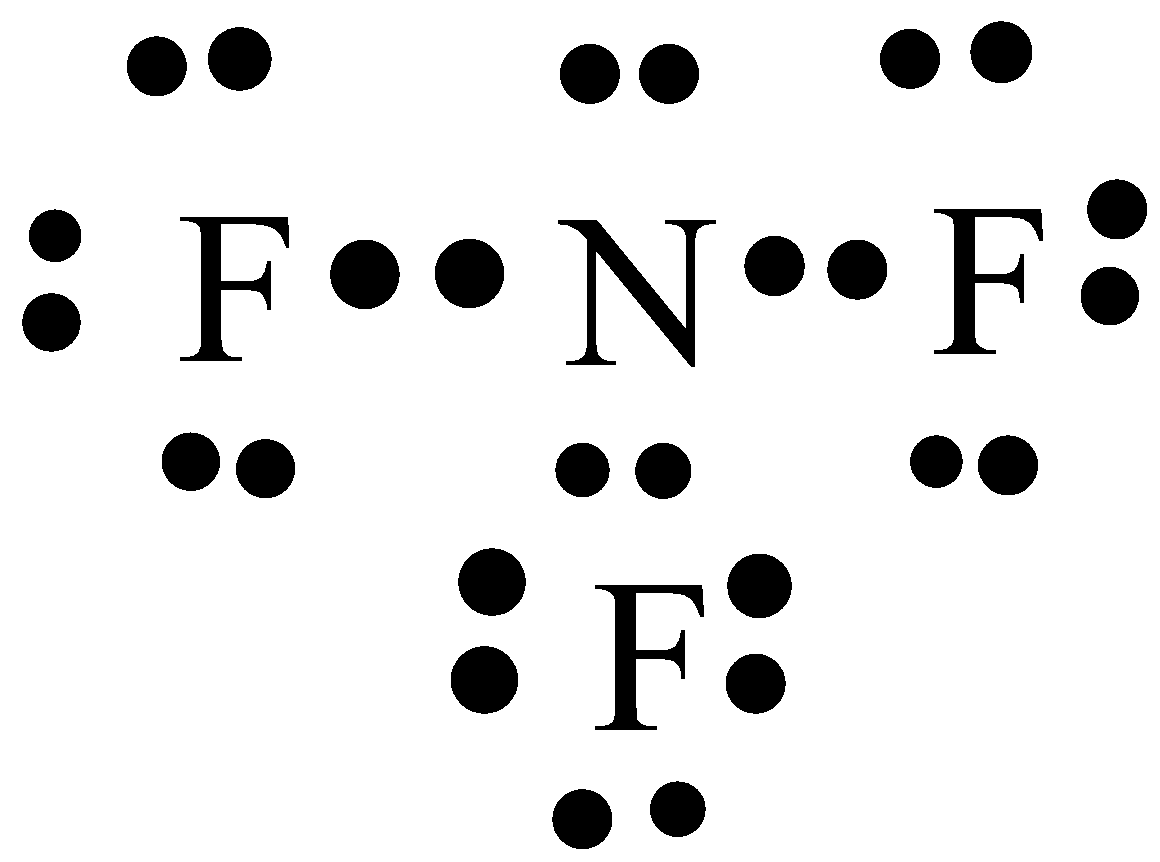
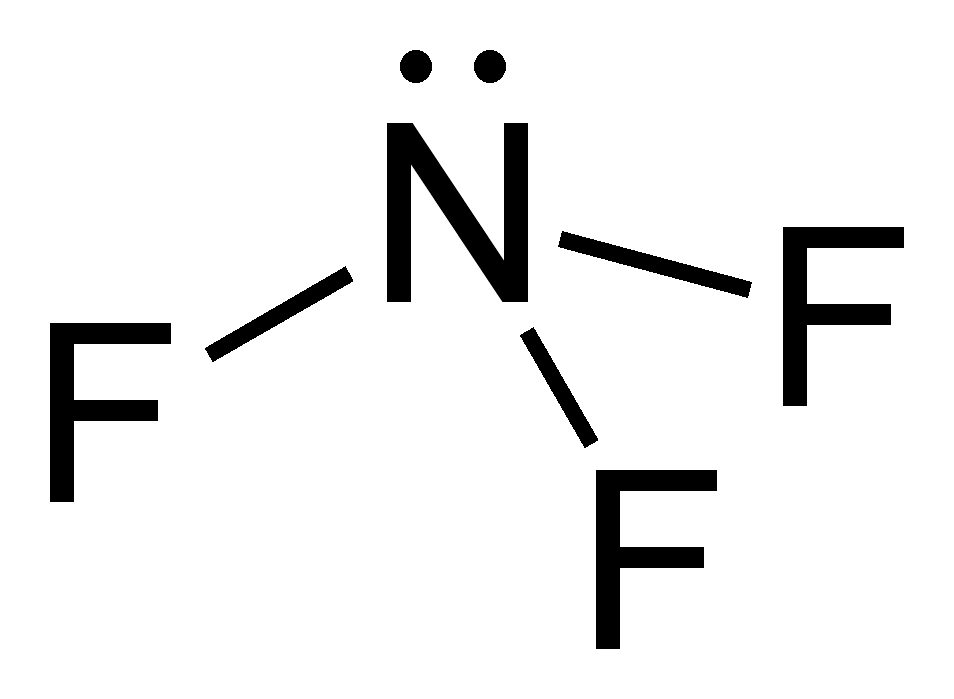
So, nitrogen is the central atom that has 1 lone pair and 3 bonded pair electrons according to the NF3 lewis dot structure. Hence the formula of NF3 becomes AX 3 N 1 So, according to the VSEPR chart , if the molecule has the formula of AX 3 N 1 , it has a molecular shape of trigonal pyramid and electron geometry of tetrahedral.
How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
formal charge on the nitrogen and oxygen atoms must add up to -1. Draw the Lewis structures and the resonance structures for NO3 -. See page 637 of your text. The negative charge generally resides on the more electronegative atom. Also note that fluorine is the most electronegative element in the periodic table.
Lewis Structure Let's look at the Lewis structure of and N2. Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). In the Lewis structure there is a triple bond between the nitrogen atoms and a non-bonding pair of electrons on each. This is consistent with the physical properties of N2. Molecular Orbitals
The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . .
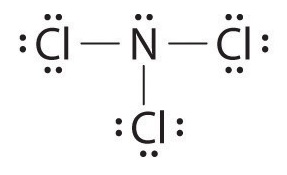
In the lewis structure of Nitrogen trifluoride (NF 3), there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps.
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
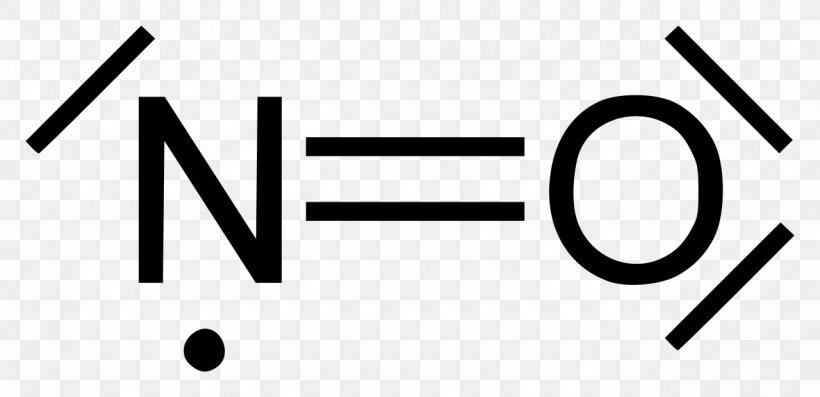
what is the Lewis structure of NO nitrogen monoxide, Lewis structures of nitrogen monoxide, Lewis electron dot structures of nitrogen monoxide, electron dot structures of nitrogen monoxide, NO Lewis structures, NO electron dot structures, NO dot structures, pi an d, for the draw, lewis no, dot structure of NO, electron dot lewis structure of NO, resonance structures of NO, ap chemistry lewis ...
How is the Lewis structure for nitrogen dioxide drawn? NO2 has 5+6+6=17 valence electrons. N is in the center and sp2 hybridized to a triangular planar shape. One O atom is double bonded and has two nonbonded electron pairs on it. The other O atom is single bonded with three nonbonded electron pairs. There is a single electron on the N atom.
Lewis Structure (electron dot diagram) for ammonia OR Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.
Drawing the Lewis Structure for N 2 ( Dinitogen or Nitrogen Gas) Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2 ). It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.
What is the Lewis structure for N2H4? N2H4 is straightforward with no double or triple bonds. In the N2H4 Lewis structure the two Nitrogen (N) atoms go in the center (Hydrogen always goes on the outside). Hydrogen (H) only needs two valence electrons to have a full outer shell. In the Lewis structure for N2H4 there are a total of 14 valence ...
Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). Click to see full answer Thereof, what are electron dot diagrams used for?


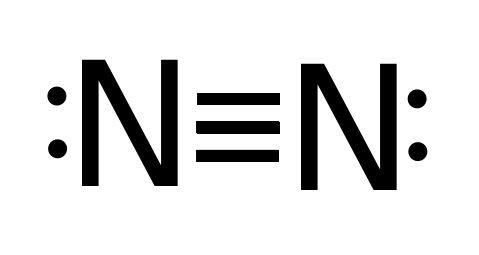
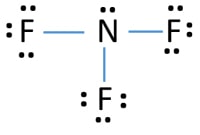
























Comments
Post a Comment