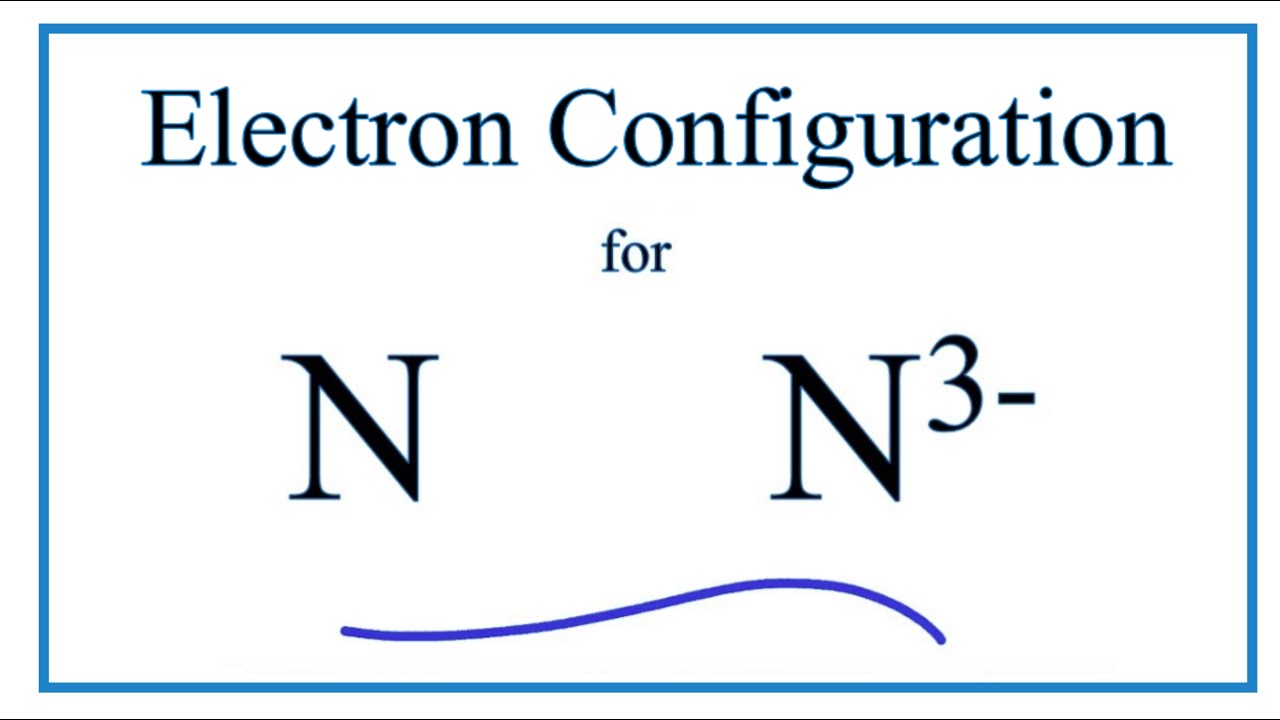
39 orbital diagram nitrogen
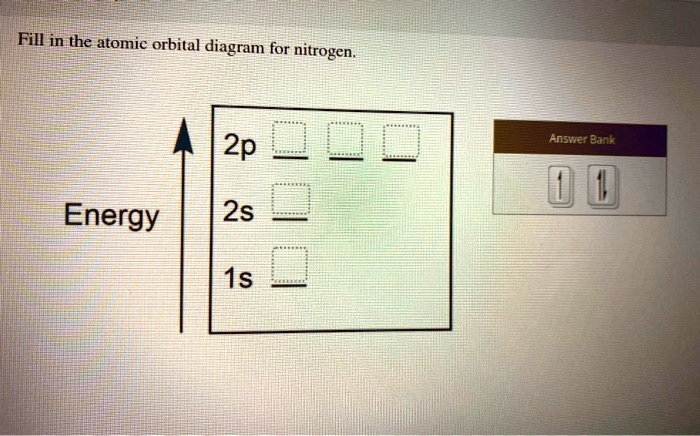
15.02.2021 · Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration February 15, 2021 by Sneha Leave a Comment Nitrogen Electron Configuration : When we talk about school subjects, then one of the major subjects which are very important for knowledge perspective is … Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
Overview. A molecular orbital (MO) can be used to represent the regions in a molecule where an electron occupying that orbital is likely to be found. Molecular orbitals are approximate solutions to the Schrödinger equation for the electrons in the electric field of the molecule's atomic nuclei.However calculating the orbitals directly from this equation is far too intractable a problem.

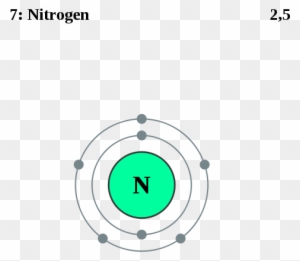
Orbital diagram nitrogen
orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and two of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule. I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c... Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures.
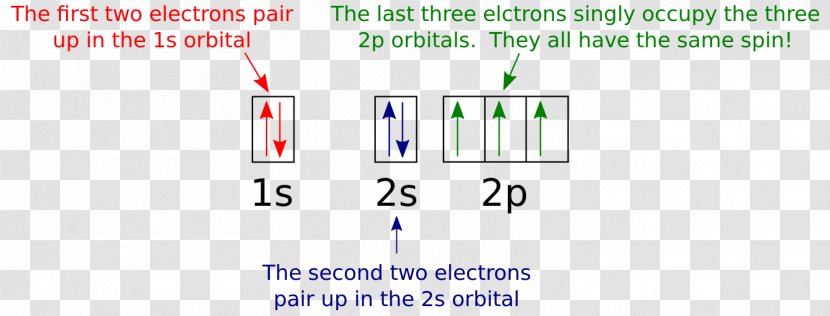
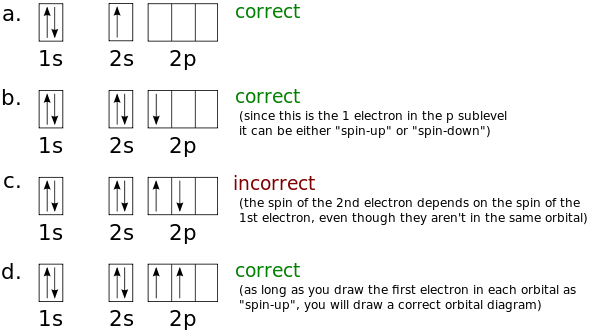
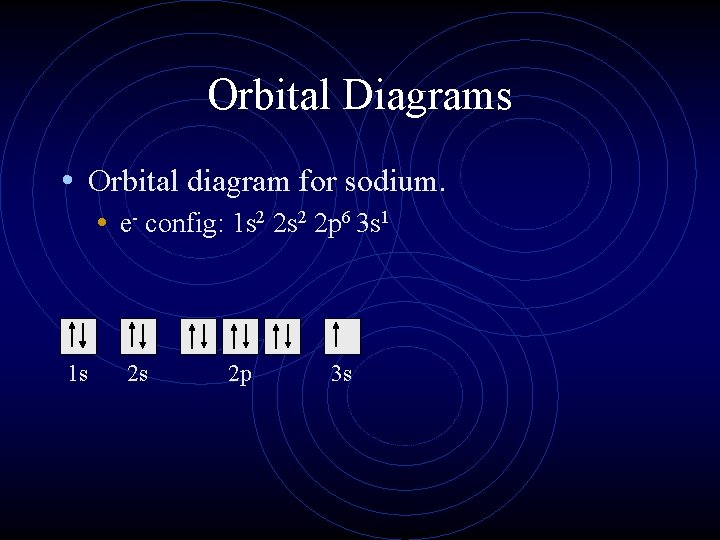
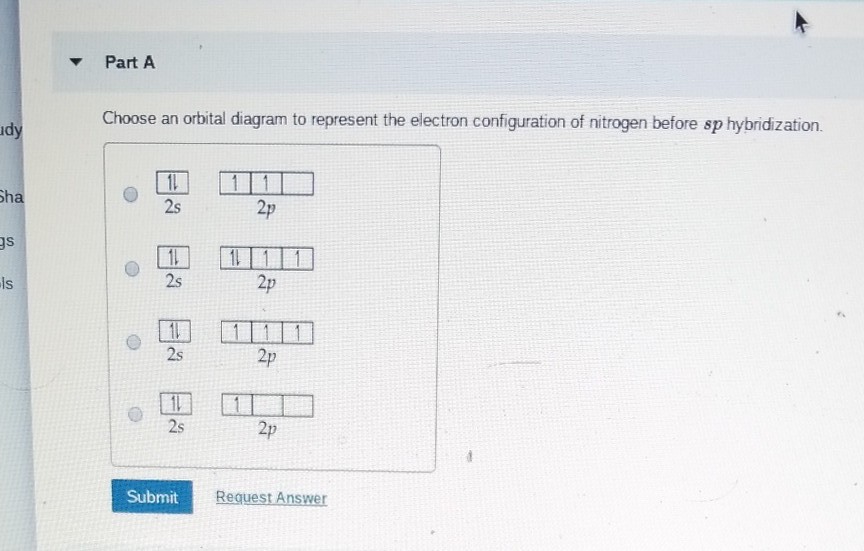
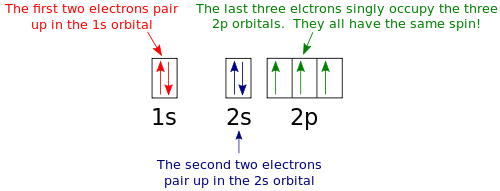
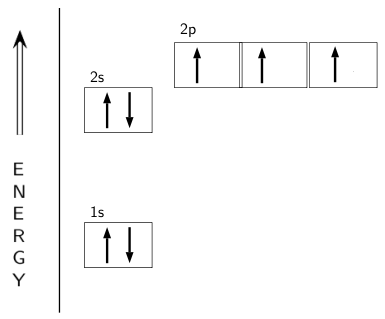
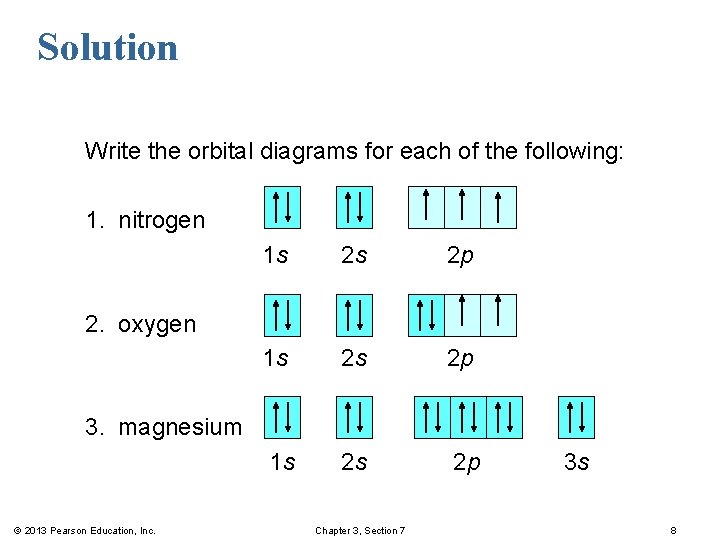
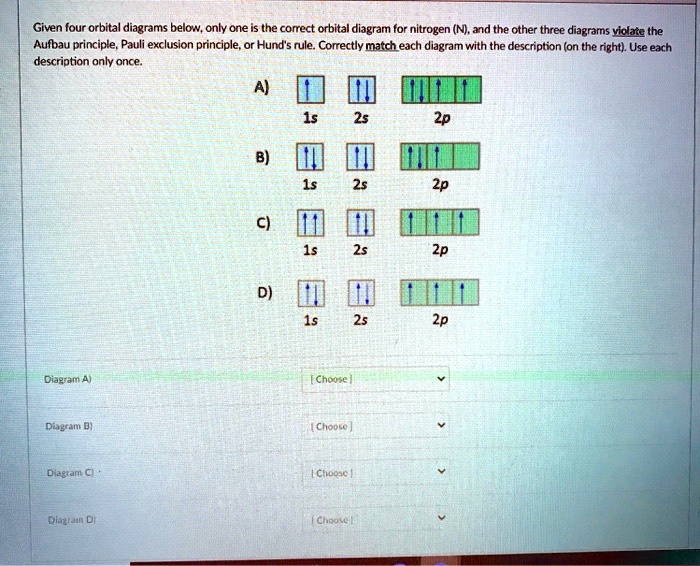
Orbital diagram nitrogen. 28.03.2018 · Orbital diagrams are like the configuration notation just introduced, except with the spins of electrons indicated. Use the Pauli exclusion principle and Hund’s rule to work out how to fill shells. The exclusion principle states that no two electrons can share the same four quantum numbers, which basically results in pairs of states containing electrons with opposite spins. Click here to get an answer to your question ✍️ Give orbital diagram of the following:nitrogen.1 answer · Top answer: Answr has image solution available for this question Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. Good morning, I'm looking for a way to compute molecular orbital diagrams using fragments or different molecules. I mean something like the diagram linked [here](https://commons.wikimedia.org/wiki/File:H2O-MO-Diagram.svg), where, instead of considering the H2 fragment and the oxygen atom I could put two fragments chosen by me or two molecules. I searched but, at the moment, I didn't find anything useful. I can use Gaussian or Orca for the calculations so if it was possible to obtain such dia...
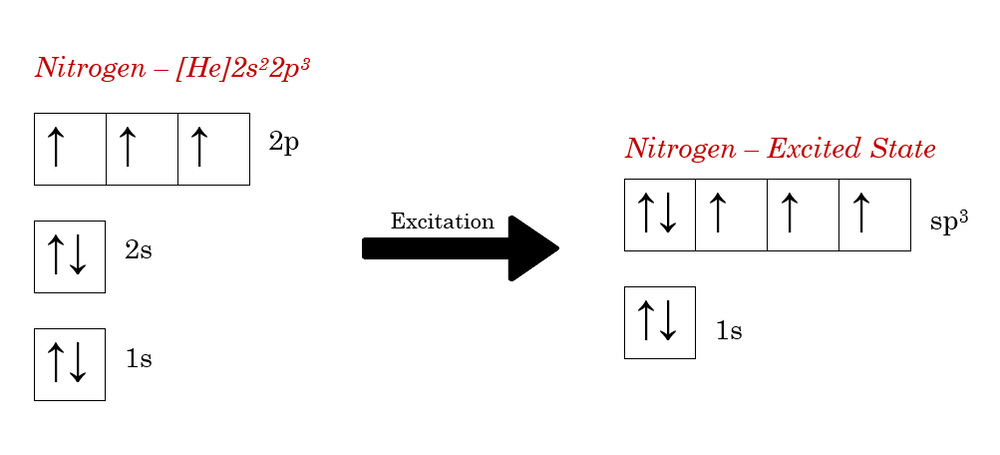
Hey Guys, ​ I was wondering if there are some databases for molexular orbital diagrams of more unusual compounds like phosphaalkenes or sulfur nitrides. I wanted to include some in a presentation ​ thanks for any help! the 2p orbital already has 3 lone electrons to bond with Hydrogen atoms, so why do they need to hybridise? And then why can't it be 3 sp2 orbitals instead of 4sp3? Atomic Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals ...1 answer · Top answer: Nitrogen From the periodic table, we can find Nitrogen which has an atomic number of 7. According to the Aufbau priniciple we have to completely... The hydrogen element is exceptional compared to the other elements in the periodic table. This article gives an idea about the electron configuration of hydrogen and orbital diagram, period and group, valency and valence electrons, bond formation, hydrogen compound formation, application of different principles. FAQs. 1.
Sorry if it's a dumb question, I'm having trouble understanding 20.02.2021 · Carbon Electron Configuration: If you guys have come across our recent article then it would be easy for you all to understand the concept.But if you are new here and looking for the information related to the carbon element and its electronic configuration, then today we will help you with some of the things and if you will be here till the last line surely you will go with some … The molecular orbital diagram representing this order of energy levels is shown in fig. Fig. No. 5 Order of Energy Levels for Boron, Carbon, Nitrogen etc. This kind of energy reversal is due to mixing of 2s and 2p orbitals where the energy difference is very close, that is, for B, C, and N … If someone were to ask me what are the angles of say, sp3 nitrogen and sp3 oxygen, should I answer that it is \~109°, or should I take into consideration the repulsion between orbitals? In that case, for example, the right answer would be \~107° for nitrogen, and \~104° for oxygen. I'm having trouble undersanding if orbital repulsion happens "before" or "after" bonds are formed. Is the geometry tetrahedrical for both? Or is it pyramidal/bent respectively?
For group, there should be a fully filled s sublevel and two electron in the outermost p sublevel. Based on how covalent bonds form with singly filled orbitals, in group14, there are only 2 singly filled orbitals respectively, how can they form the expected 4 bonds? Or does the electron from s move to p to give 4 singly filled orbitals? If that is the case, why does this happen for no reason?
Can you provide any evidence beyond a quick google search (i.e., literature) as to whether the M.O. diagram for nitrogen monoxide has the valence sigma and pi orbitals switched (e.g., as in the M.O. for molecular nitrogen) or is the sigma lower in energy than the pi orbitals?
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures.
I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c...
orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and two of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule.














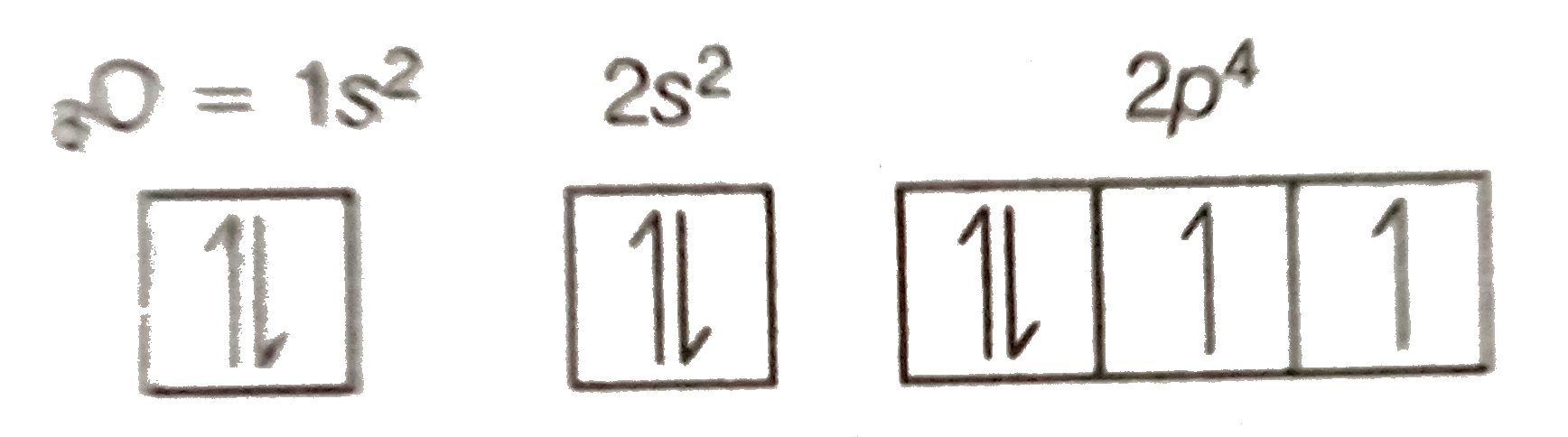






Comments
Post a Comment