40 bn mo diagram
MOT(V) CN, BN, CN- आण्विक कक्षक चित्र - YouTube ¤ Molecular Orbital diagram of COhttps://youtu.be/8mufOTgvagU¤Molecular Orbital diagram of NO , NO+, NO- ¤ S-P mixing OF ORBITALS ... How to draw a BN molecular orbital diagram class 11 ... Hint: An orbital diagram explains how the orbitals of the atoms in the molecule are going to split when they are involved in bonding to form a molecule.Molecular orbitals are different from the atomic orbitals. Complete answer: - In the question it is given to draw the molecular orbital diagram for BN molecules.
Boron(B) electron configuration and orbital diagram Boron (B) excited state electron configuration and orbital diagram. Then correct electron configuration of boron in the ground state will be 1s 2 2s 2 2p x1. The valency of an element is determined by electron configuration in the excited state. When the boron atom is excited, then the boron atom absorbs energy.
Bn mo diagram
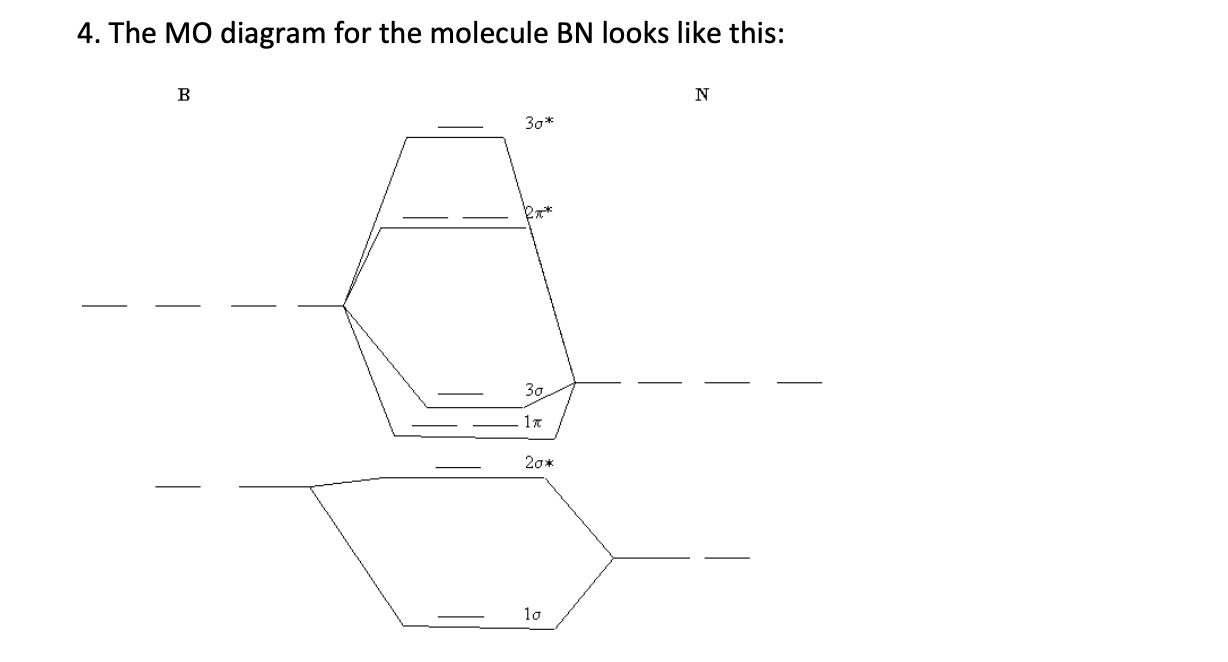
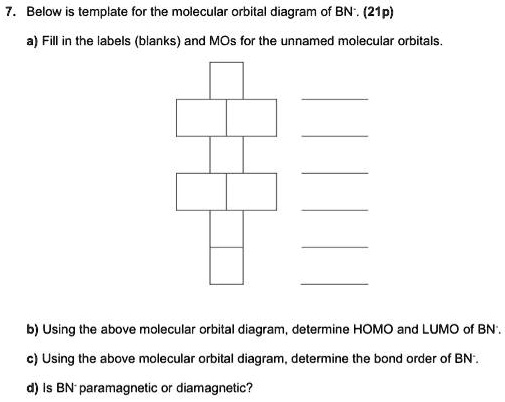
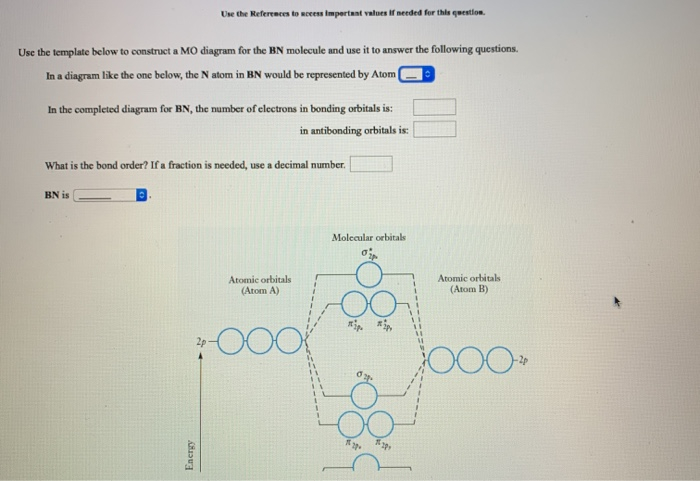
Molecular Orbital Diagram Maker ©2021 Prof Adam J Bridgeman | close window : ©2021 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window Solved 4. The MO diagram for the molecule BN looks like ... The MO diagram for the molecule BN looks like this: B N 30* 30 17 20 10 Populate the diagram with electrons and write down (2 points each): 4.1 The MO electronic configuration of BN 4.2 Number of unpaired electrons in BN 4.3 Bond order of BN Now imagine that you have added an extra electron to produce BN molecular ion 4.4 The MO electronic ... Molecular Orbital Diagram Of Bn - Pinterest Molecular Orbital Diagram Of Bn. Find this Pin and more on chemistry by Kallie Lively. Molecular Shapes. Energy Level. Wikimedia Commons. Mathematics. Chemistry. Bond. Diagram.
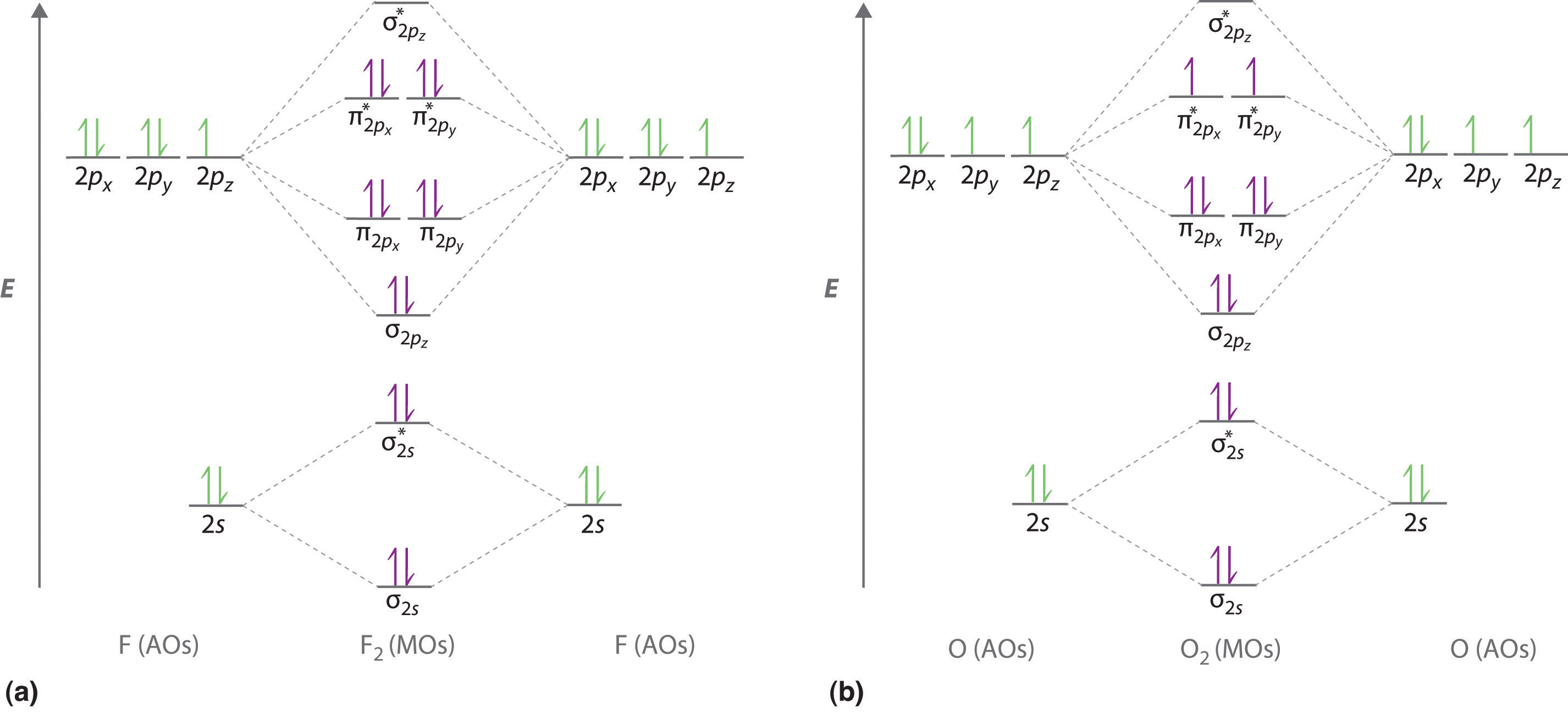
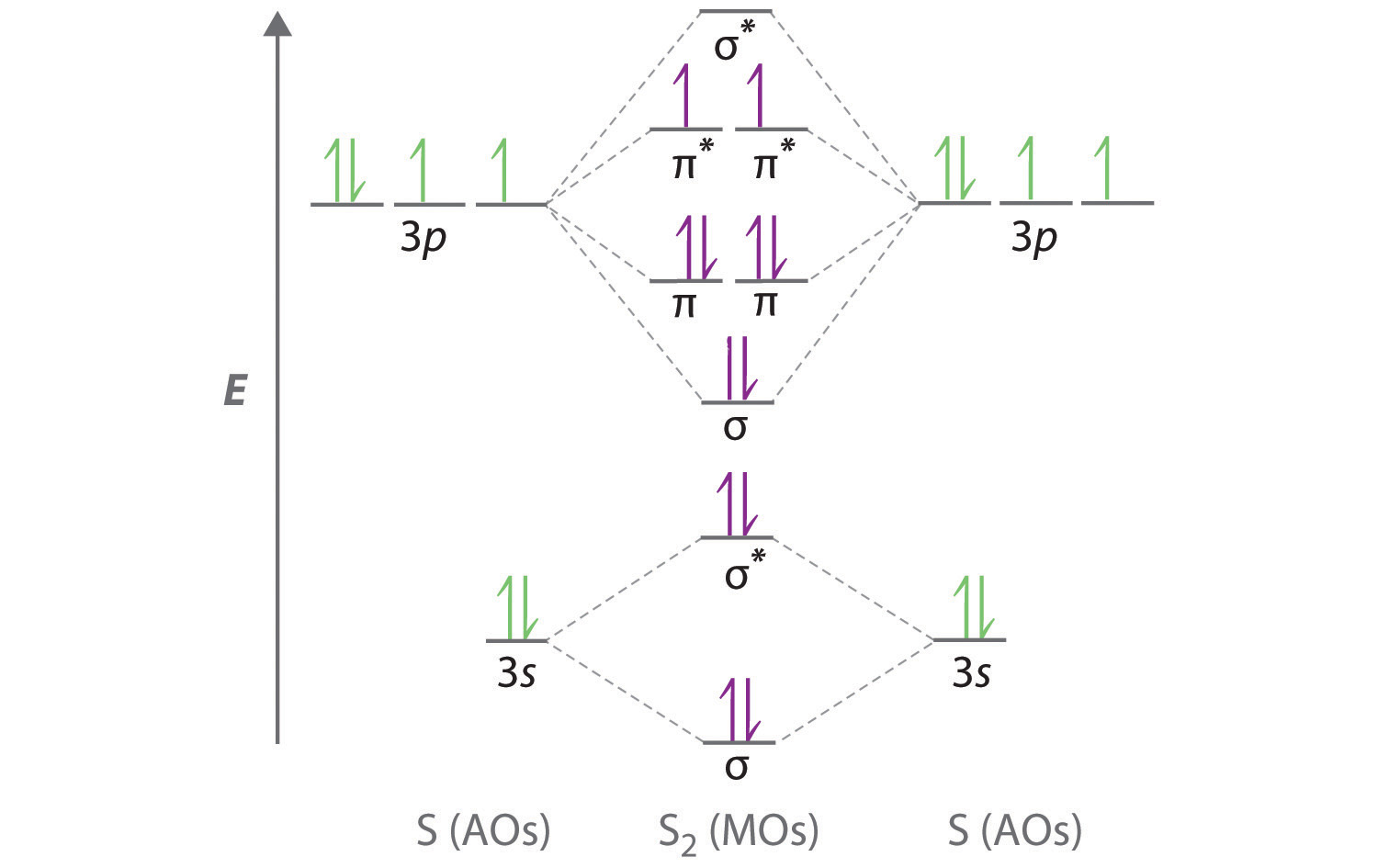
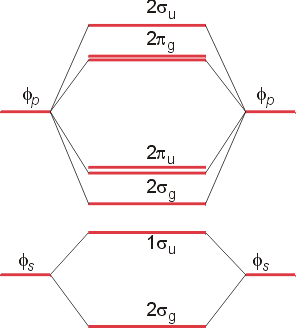
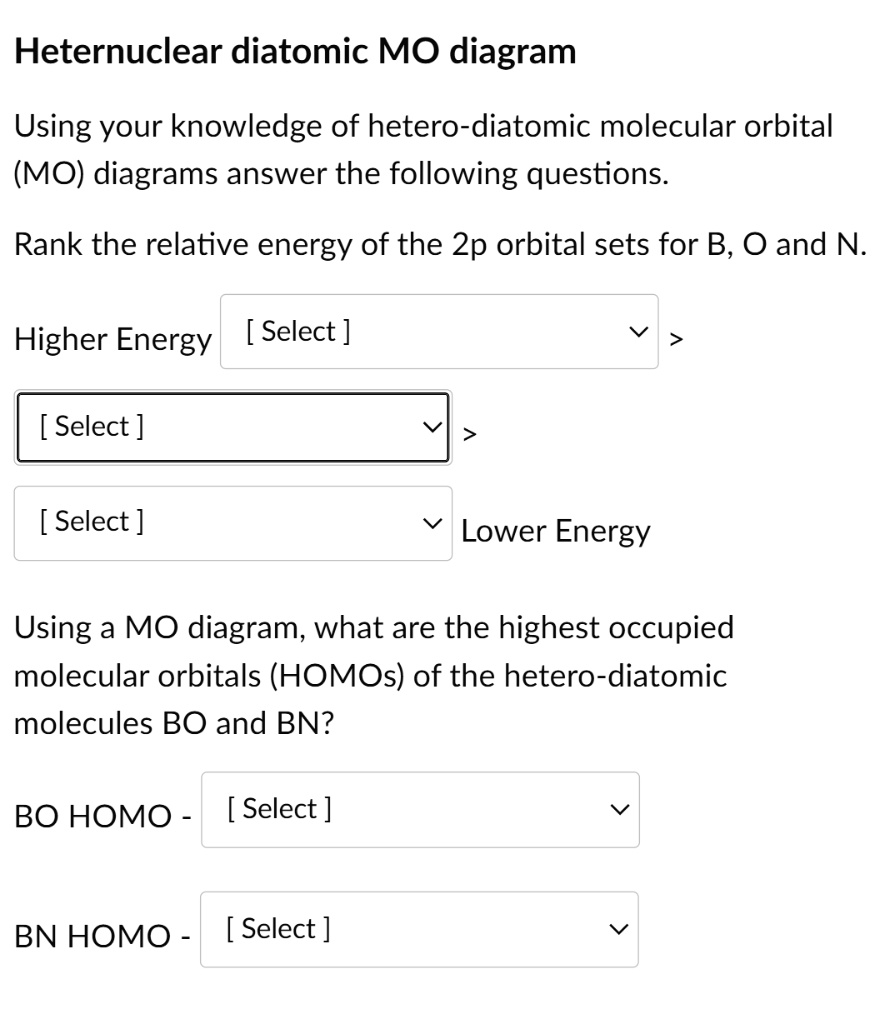
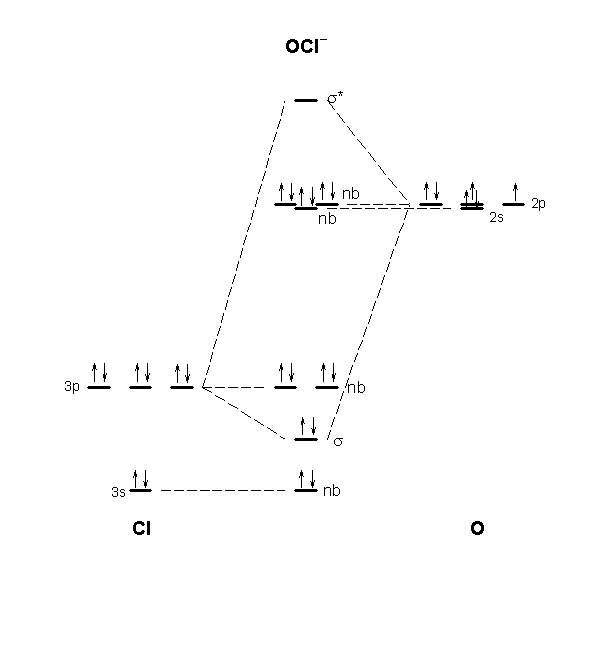
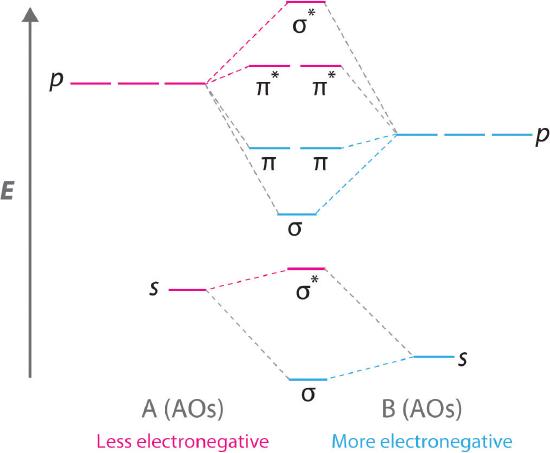
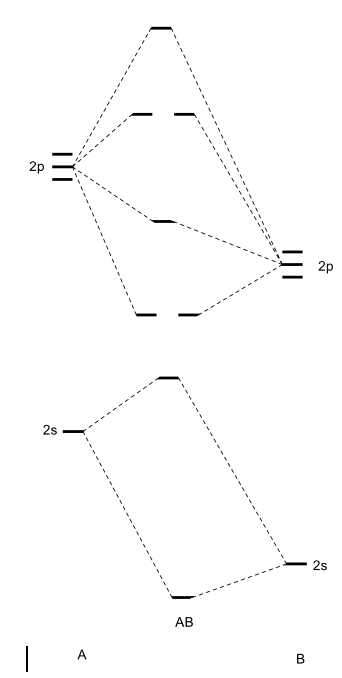
Bn mo diagram. MO Theory: Heteronuclear Diatomic Molecules - Chemistry ... Practice: Apply Molecular Orbital Theory to determine the MO orbital diagram for the CF + ion. Practice: Using a MO diagram, write the electron configuration for the BN molecule? Progress PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine How to draw a BN molecular orbital diagram? | Socratic Dec 11, 2017 · This is the general MO diagram you need to fill with the valence electrons of BN Boron has 3 valence electrons, and nitrogen has 5 valence electrons, this makes 8 electrons. You have to start filling the orbitals from those with lowest energy to those with higher energy. So, 2 electrons on σ2s , two electrons on σ∗2s, two electrons on σ2p . You have now 2 electrons left, and two orbitals ... MO diagram of BN - The Student Room Also, in the MO diagram of CO, is 2 sigma molecular orbital a bonding orbital, while the 3 sigma is an anti-bonding orbital? BN is much similar to the MO of the hypothetical diatomic carbon. you are better off if you can draw MO of the diatomic carbon and shift slightly the two initial AOs to adjust for different Zeff, hence electronegativity ...
High-resolution XPS spectra of a Mo 3d of Ag/MoS2 film; b ... Download scientific diagram | High-resolution XPS spectra of a Mo 3d of Ag/MoS2 film; b Mo 3d of Ag/BN/MoS2 film; c S 2p of Ag/MoS2 film; d S 2p of Ag/BN/MoS2 film; e N 1 s of Ag/BN/MoS2 film; and ... Use The Mo Diagram Provided Below To Answer The Following ... Using your BN molecular orbital diagram (in #3) as a guide, draw a. [GET] Use The Mo Diagram Provided Below To Answer The Following Questions . This MO is called the bonding orbital and its energy is lower than that of the original atomic orbitals. A bond involving molecular orbitals which are symmetric ... What is the bond order of the diatomic molecule BN and is ... I spent some time looking on the web for the Lewis structure for BN but had no luck. I would do it this way. B:::N: B has 3e and N has 5 and 8 total. The structure I have drawn has zero formal charge on both B and N. The structure I have drawn has no unpaired eletrons; therefore, it is not paramagnetic. Molecular Orbital Theory - Purdue University Valence Bond Model vs. Molecular Orbital Theory . Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory.. The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond.
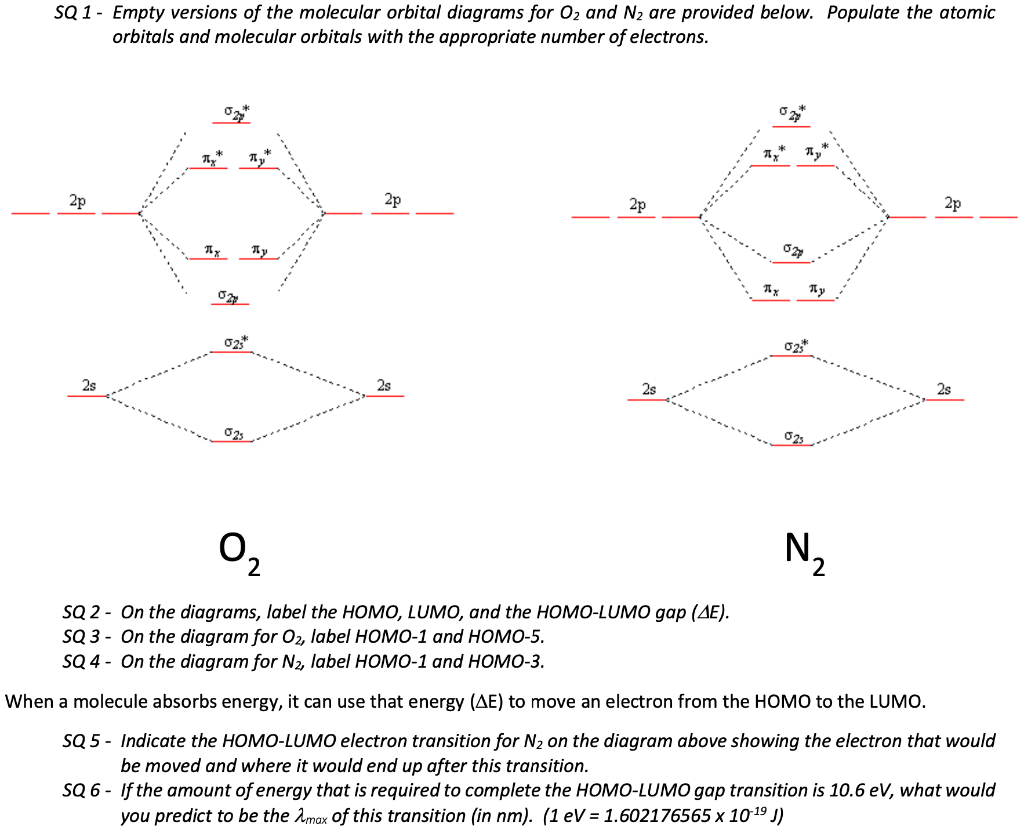
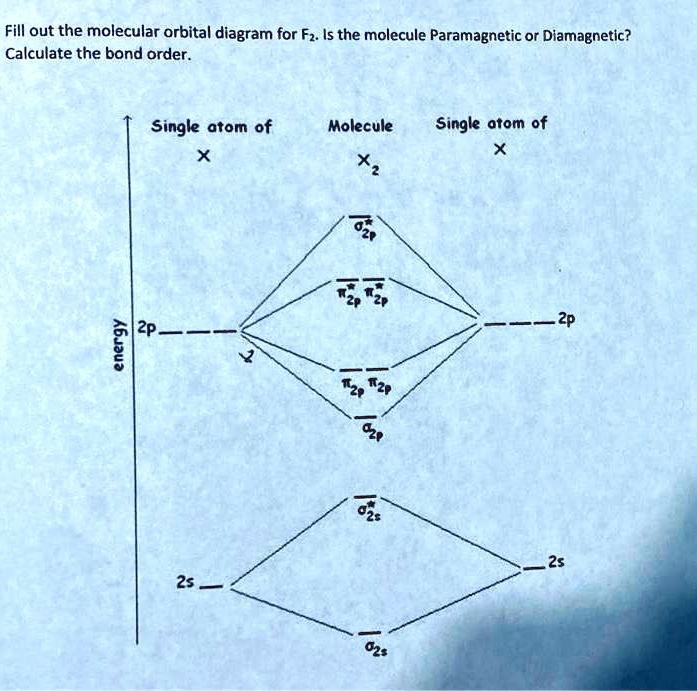
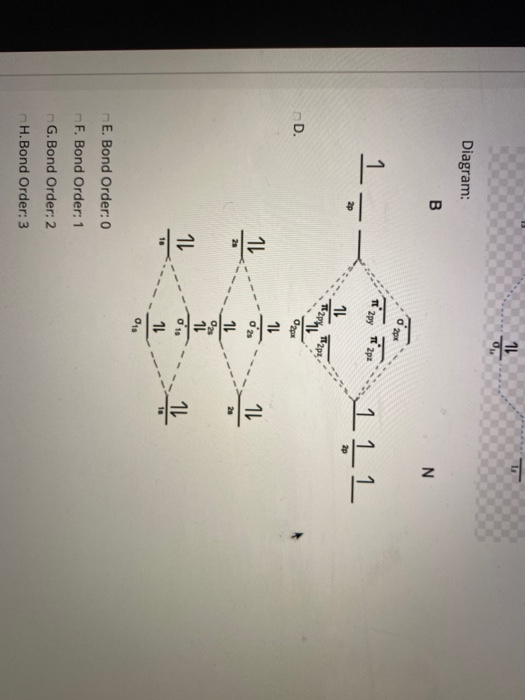
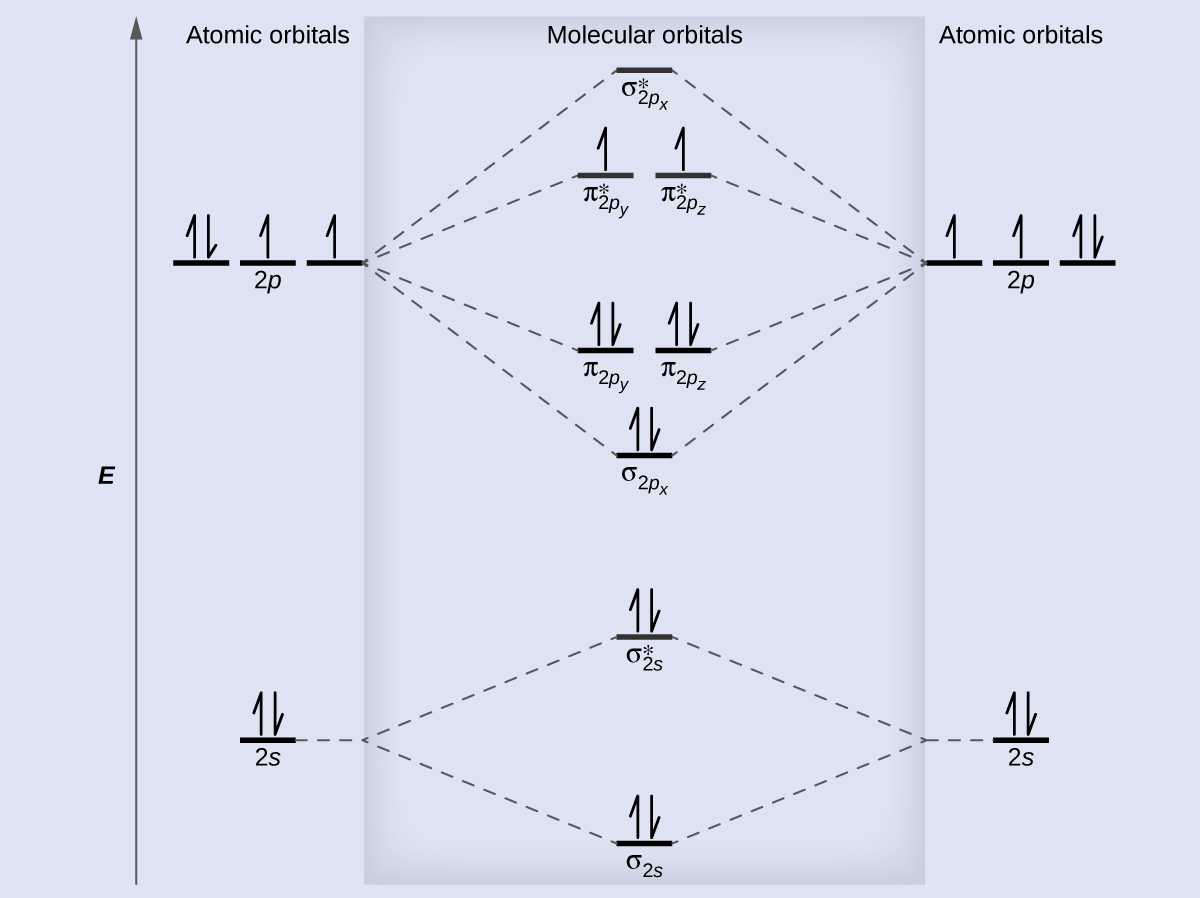
Homework 8 Flashcards - Quizlet Complete the MO diagram (below) to determine if O₂ is paramagnetic or diamagnetic. A) paramagnetic B) diamagnetic. A) Paramagnetic. The molecular orbital energy diagram for F₂ is shown below. Based on this diagram, what is the bond order of F₂? 1. Homework4 - MSE 110 Problem Set 4 Problem 1 ... - Course Hero Problem 2 Build it s MO diagram of the BN molecule. Do you expect the BN molecule to be paramagnetic or diamagnetic? (8pts) [Answer A or B] A- paramagnetic B- diamagnetic Ans: The BN molecule has a total of 12 electrons. Following the Hunds rule two electrons will occupy both π orbitals with their spin up. This will produce a net magnetization of the ion, which is therefore paramagnetic. SOLVED: Mo diagram of BN we're being asked to explain the giving diagram and our diagram shows us that effort four equals 11 corresponds to the oId a pair for 11. Well, if we think about the definition of a function we have f of X is equal to why? Well, wouldn't that correspond to the order pair X Y And indeed it would. So our input, which in this case is four, would be the X value of the order Pair in the output ... MO Diagrams - GitHub Pages #3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron.
Solved 4. The MO diagram for the molecule BN looks like ... The MO diagram for the molecule BN looks like this: 5. The total energy eigenvalues for the hydrogen atom are given by En=-e/(81 Egaon) with n= 1, 2, 3... etc. and the three quantum number associated with the H-atom orbitals are: 1) the principal quantum number n, 2) the angular momentum quantum number 1 and 3) the magnetic quantum number m.
Quiz3-2021f.pdf - Quiz#3 Chem 4120\/6120 December 2nd 2021 ... The MO diagram for the molecule BN looks like this: Populate the diagram with electrons and write down: 2.1 The MO electronic configuration of BN (2 points) 2.2 Number of unpaired electrons in BN (2 points) 2.3 Bond order of BN (2 points) 2.4 The Slater determinant of BN (6 points) Now imagine that you have added an extra electron to produce BN ...
Answered: Complete the MO diagram (below) to… | bartleby Maps Question 17 of 20 Submit Complete the MO diagram (below) to determine if BN is paramagnetic or diamagnetic. Op A) paramagnetic B) diamagnetic Os Os LO C 12:27 PM O Search for anything 83% 11/20/2020 +.
Module Two Chem 101 Problems Flashcards - Quizlet Module Two Chem 101 Problems. TestNew stuff! Carbon dioxide is a _____ compound composed two types of _____ atoms. Carbon dioxide is a molecular compound composed of two oxygen atoms with covalent double bonds to a central carbon atom. Both C and O are to the right of the "staircase" on the periodic table, as nonmetals.
MO Diagram of BN Sp 5 A4 - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Molecular Structure Practice Problems Answers (c) The MO diagram for BN can be constructed, similar to that of the homonuclear diatomics: The electron configuration becomes 1σ 2 2σ* 2 1π 4, the number of unpaired electrons is 0, and the bond order is 2. Other MO diagram constructions can give different electron configurations and bond orders, but should always give no unpaired spins.
41 boron molecular orbital diagram - Diagram For You Dec 16, 2021 · This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electrons, and nitrogen has 5 valence electrons, this makes 8 electrons. You have to start filling the orbitals from those with lowest energy to those with higher energy. So, 2 electrons on σ 2 s, two electrons on σ 2 s ∗, two electrons on ...
molecular orbital theory - How can BN be paramagnetic ... Nov 20, 2014 · This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electrons, and nitrogen has 5 valence electrons, this makes 8 electrons. You have to start filling the orbitals from those with lowest energy to those with higher energy. So, 2 electrons on σ 2 s, two electrons on σ 2 s ∗, two electrons on ...
Molecular Orbital Diagram Of Bn - Pinterest Molecular Orbital Diagram Of Bn. Find this Pin and more on chemistry by Kallie Lively. Molecular Shapes. Energy Level. Wikimedia Commons. Mathematics. Chemistry. Bond. Diagram.
Solved 4. The MO diagram for the molecule BN looks like ... The MO diagram for the molecule BN looks like this: B N 30* 30 17 20 10 Populate the diagram with electrons and write down (2 points each): 4.1 The MO electronic configuration of BN 4.2 Number of unpaired electrons in BN 4.3 Bond order of BN Now imagine that you have added an extra electron to produce BN molecular ion 4.4 The MO electronic ...
Molecular Orbital Diagram Maker ©2021 Prof Adam J Bridgeman | close window : ©2021 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window




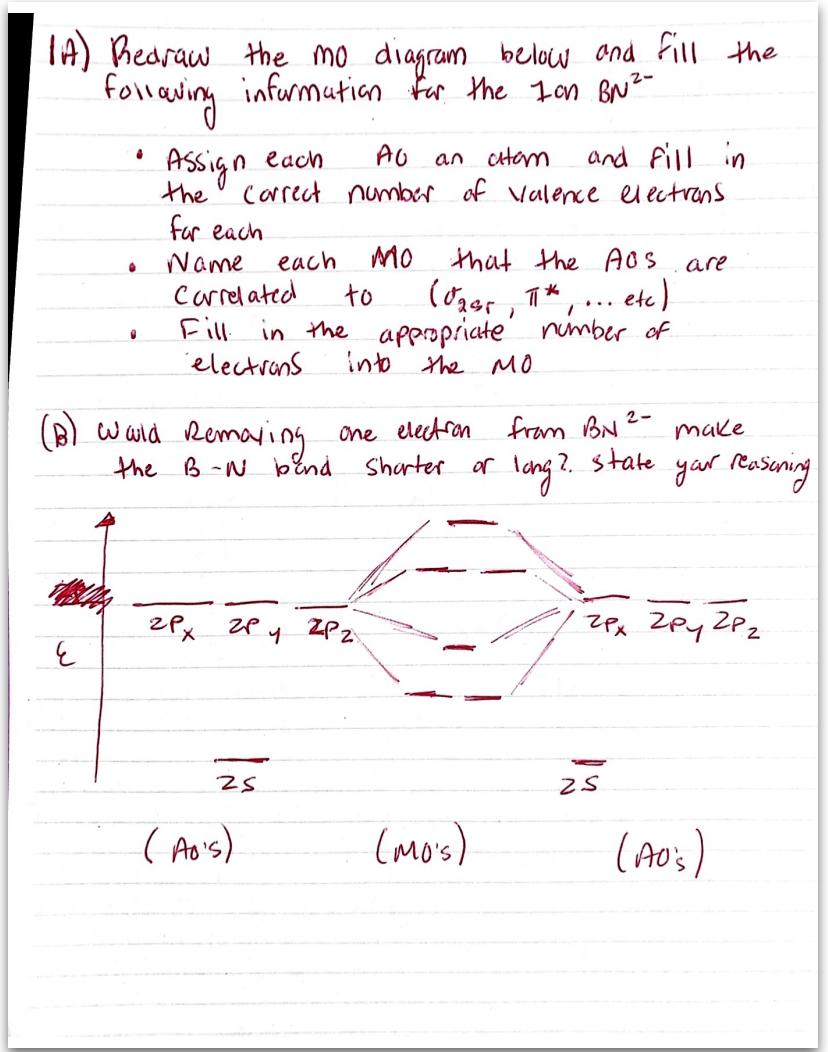
















Comments
Post a Comment