42 orbital diagram for nitrogen
What is the electron configuration and orbital diagram of ... What is the electron configuration and orbital diagram of nitrogen? In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
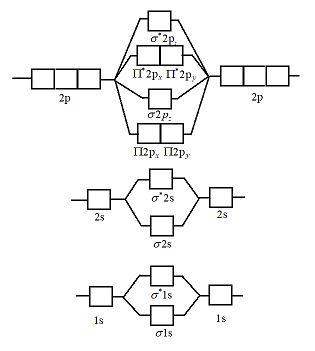
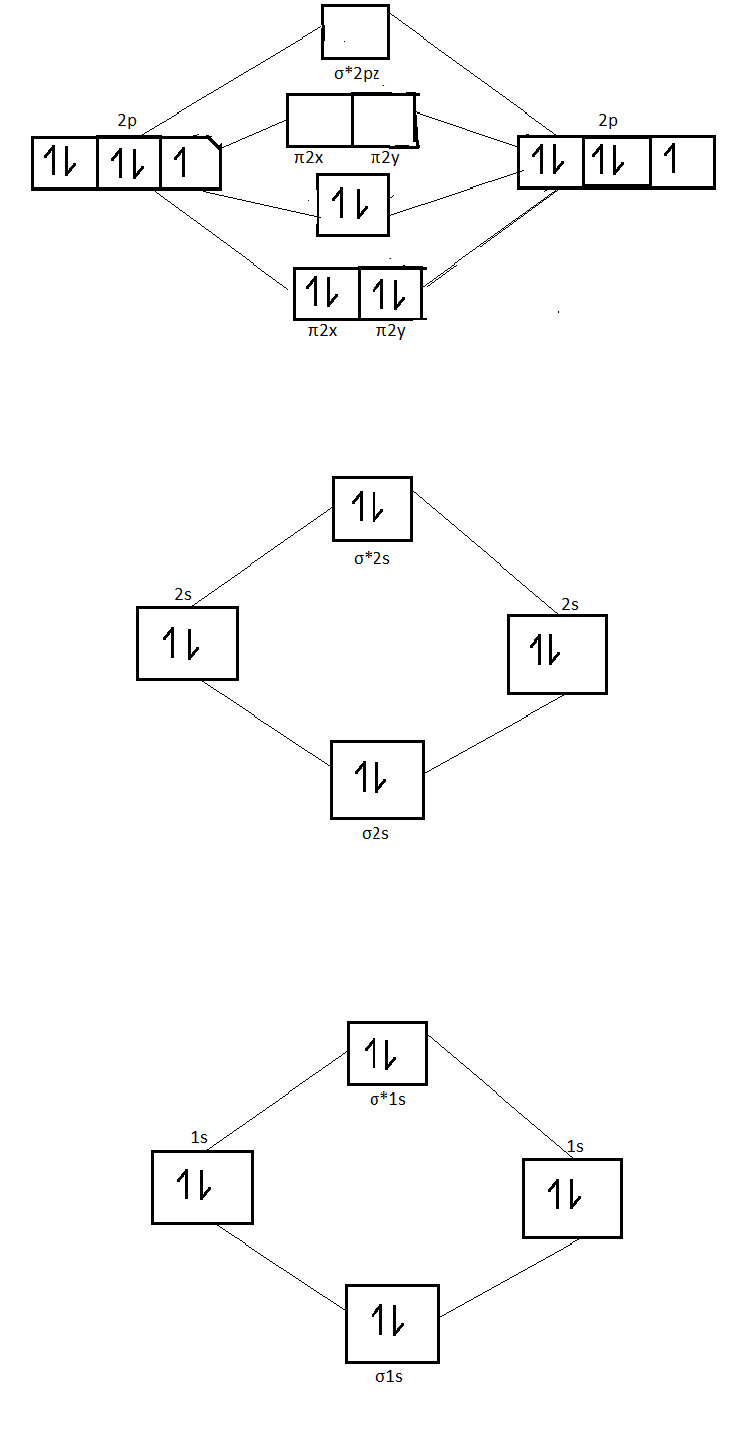
8 - Drawing Molecular Orbital Diagrams — Flux Science Molecular orbital diagram of diatomic nitrogen. Homonuclear molecular orbitals are formed between two elements that are the same, meaning that they are naturally symmetrical and will perfectly overlap. However, before we fill out this diagram, Compare this MOD to the one above, particularly in the 2p region.

Orbital diagram for nitrogen
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or... 1.4: Electron Configurations & Electronic Orbital Diagrams ... Example 2: Oxygen and Nitrogen. If we look at the correct electron configuration of the Nitrogen (Z = 7) atom, ... The electron configuration for sulfur is 1s 2 2s 2 2p 6 3 s 2 3p 4 and can be represented using the orbital diagram below. Exercises . Write the electron configuration for phosphorus and draw the orbital diagram.
Orbital diagram for nitrogen. Orbital Diagram For Nitrogen - Free PDF eBook Orbital Diagram For Nitrogen Free PDF eBooks. Posted on July 30, 2015. MO Diagrams for O2 and N2 - U of L Class Index. Drawing MO diagrams - always same number of MOs as AOs. ... N2 σ2s σ*2s σ* 2p π*2p π2p σ2p smaller ΔE. N2. Diamagnetic. Bond order of 3 (N≡N). Transition Metal Review.pdf. Create the atomic orbital diagram for nitrogen. - Clutch Prep Create the atomic orbital diagram for nitrogen. Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems Q. Construct the orbital diagram of each atom or ion.TiTi2+Ti4+ Q. Write the corresponding electron configuration for the following pictorial ... Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s. Nitrogen Bohr Model - How to draw Bohr diagram for ... Electron dot diagram of a Nitrogen atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Nitrogen, we got to know, it has 5 valence electrons. So, just represent these 5 valence electrons around the Nitrogen atom as a dot.
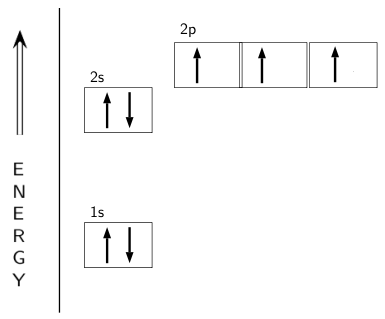
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... Nitrogen Orbital diagram, Electron configuration, and ... What is the orbital diagram for Nitrogen (N)? The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest three electrons in 2p orbital. What is electron configuration of nitrogen and copper using s ... What is electron configuration of nitrogen and copper using s, p, d, and f notation and draw its orbital diagram?2 answers · 4 votes: Nitrogen : Atomic number is 7 Electronic configuration of Nitrogen is 1s2 2s2 2px1 ... Oxygen(O) electron configuration and orbital diagram Oxygen(O) is the 8th element in the periodic table and its symbol is 'O'. This article gives an idea about the electron configuration of oxygen and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this.
Orbital Filling Diagram For Nitrogen - wiringall.com In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom. What is the atomic orbital diagram for nitrogen? Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). Furthermore, what is the configuration of nitrogen? [He] 2s2 2p3 Likewise, people ask, what is the orbital diagram for phosphorus? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. NO2 Lewis Structure, Molecular Geometry, Hybridization ... The six electrons present in the 1s orbital do not take part in bonding, therefore will play the role of non-bonding orbitals. The two electrons of 1s2 in the Nitrogen atom take part in σ2s MO. The oxygen atoms contribute to 2 lone pairs each. The remaining electrons in p orbitals of N and O form the σ2px, 𝜋2py, 𝜋2pz, and σ*2s. Conclusion Draw the molecular orbital diagram of N2N2 + N2 Write ... Electrons of nitrogen are to be filled in this diagram. Left side represents the configuration of one atom of nitrogen molecule and the right side represents the second atom of nitrogen molecule. Atomic number of nitrogen is seven. Therefore in ${N_2}$ there are a total fourteen electrons. Molecular orbital diagram of ${N_2}$ is shown below:
Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... What is the Orbital Diagram For Nitrogen? When we talk about the orbital diagram, we first need to understand what exactly it means. Therefore, during exams, the student can expect questions related to this topic so it is important that the students must go through it.
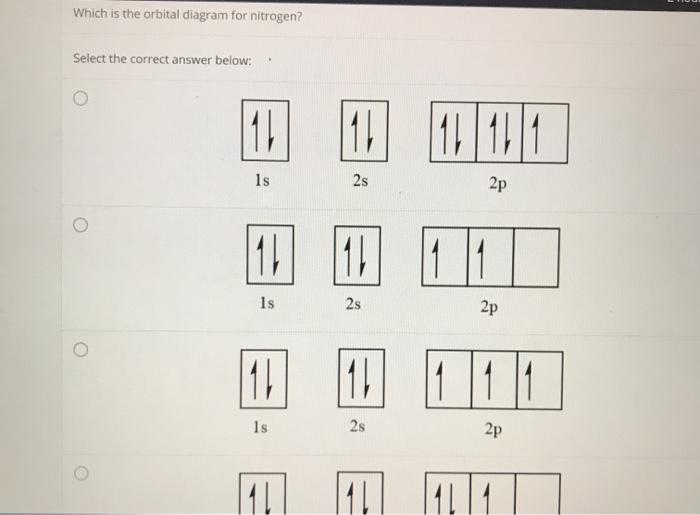
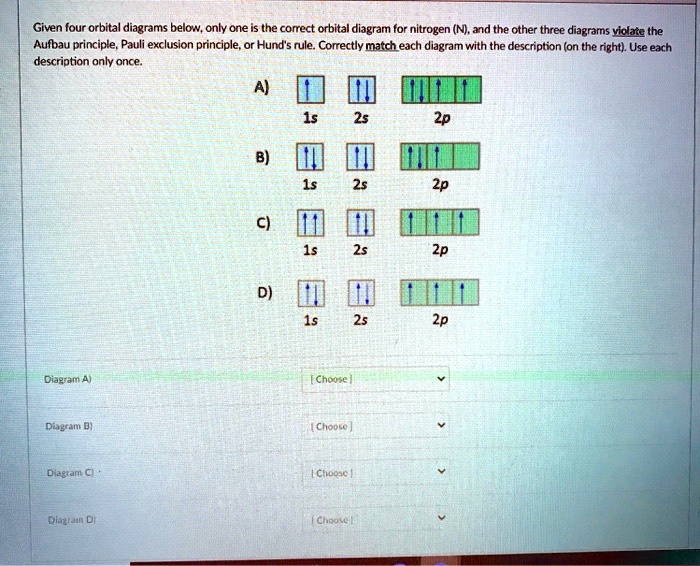
Give orbital diagram of the following:nitrogen - Toppr The ground state electronic configuration of nitrogen is as shown in diagram. Reason. Electrons are filled in orbitals as per aufbau principle, Hund's rule of ...1 answer · Top answer: Answr has image solution available for this question
Molecular Orbital Energy Diagram of N2 (Nitrogen molecule ... Molecular Orbital Energy Diagram of N2 (Nitrogen molecule) Moed diagram of N2 Moed of N2Hi everyone! Welcome to M V L N M RAJU Chemistry ClassMolecular Orbit...
Molecular orbitals in Nitrogen - ChemTube3D Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Nitrogen CONTROLS Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.
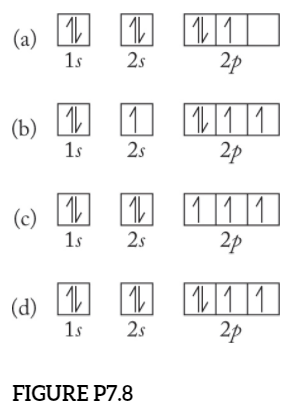
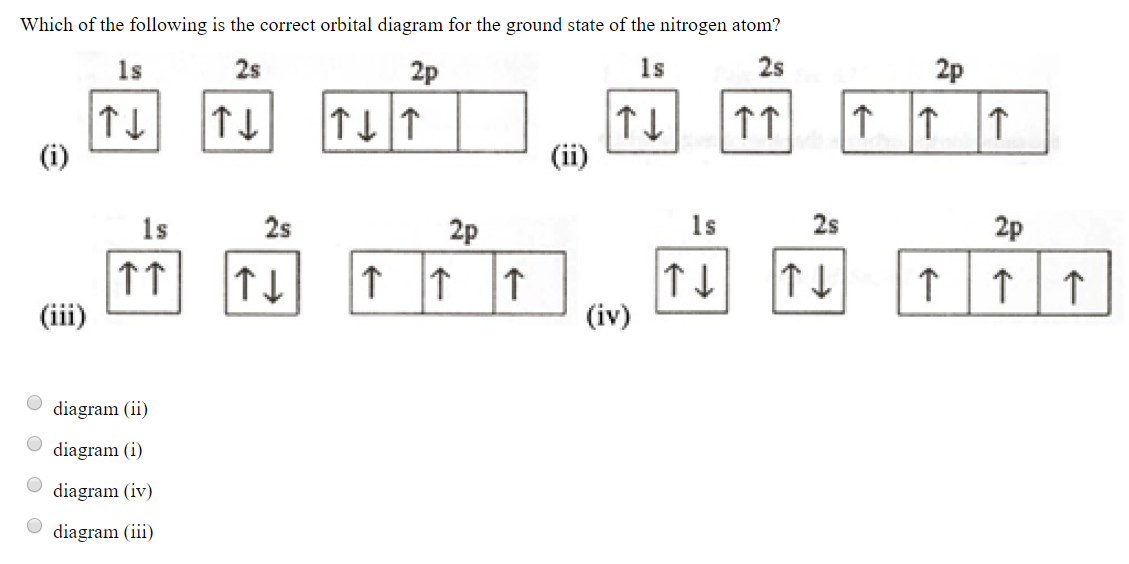
[ANSWERED] Which Of The Orbital Diagrams Represent(S) An ... Answer Electronic configuration of nitrogen in ground state is 1s2 2s2 2p3 or 1s2 2s2 2px1 2py1 2pz1. Hence, in excited state one of the 2s electron will jump to 2p orbital,so the excited state electronic configuration should be 1s2 2s1 2px2 2py1 2pz1. I BEG you
Molecular Orbital Diagram of Nitrogen Molecule - Nature of ... Molecular Orbital Diagram of Nitrogen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET...
N2+ Mo Diagram - schematron.org Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure.
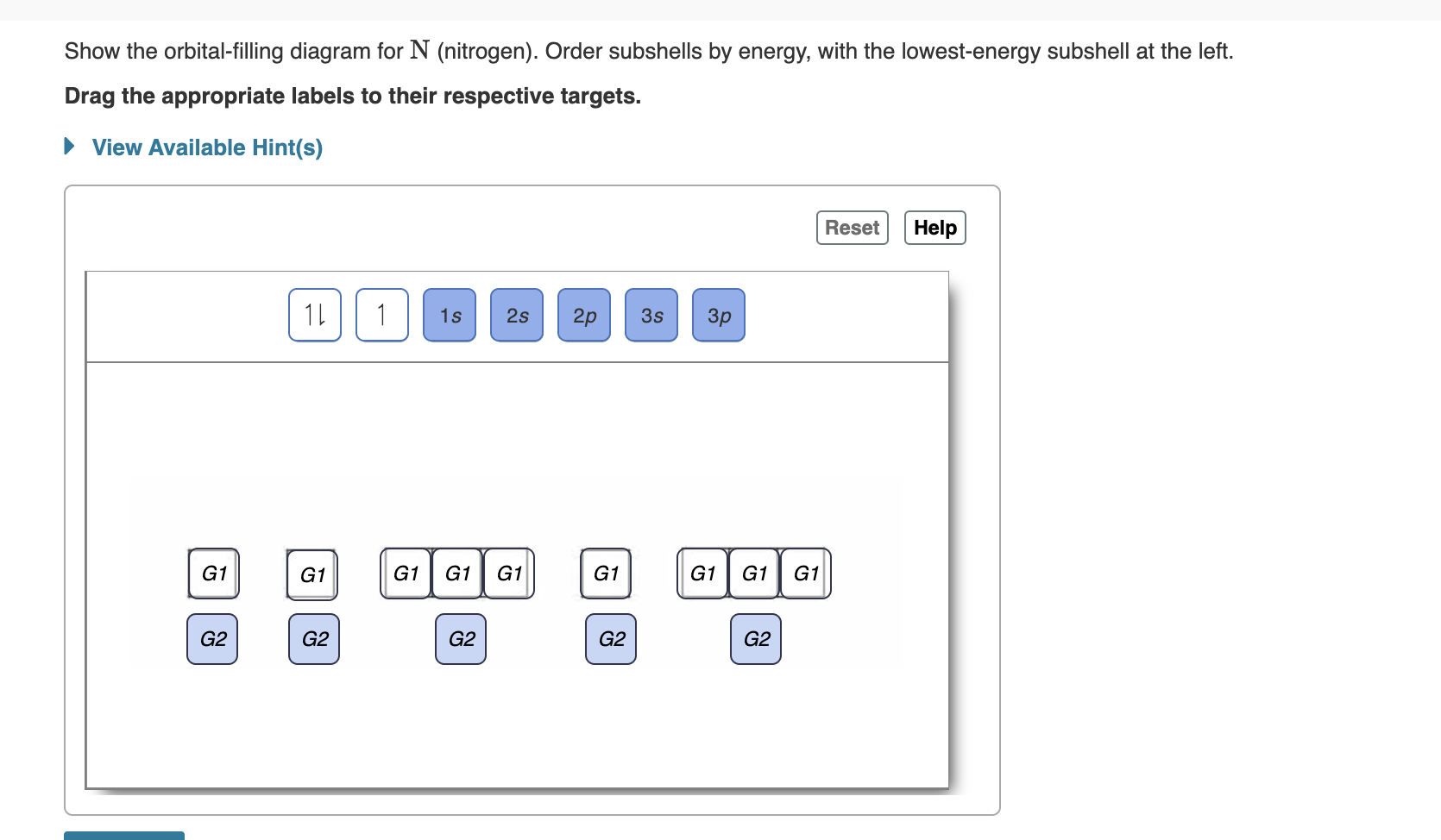
Electron Configuration for Nitrogen (N) - TerpConnect Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.
Orbital Filling Diagram For Nitrogen - schematron.org 02.09.201802.09.20186 Commentson Orbital Filling Diagram For Nitrogen Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.
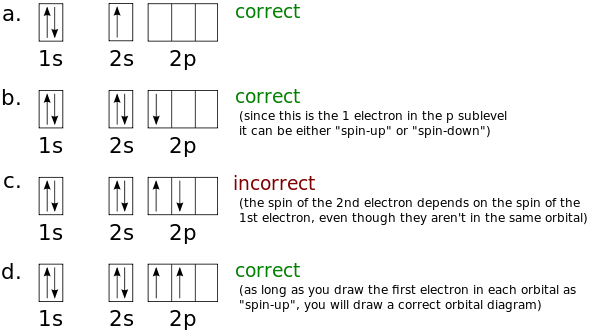
Nitrogen(N) electron configuration and orbital diagram Nitrogen (N) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
What is the atomic orbital diagram for nitrogen? | Study.com Atomic Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals ...1 answer · Top answer: Nitrogen From the periodic table, we can find Nitrogen which has an atomic number of 7. According to the Aufbau priniciple we have to completely...
1.4: Electron Configurations & Electronic Orbital Diagrams ... Example 2: Oxygen and Nitrogen. If we look at the correct electron configuration of the Nitrogen (Z = 7) atom, ... The electron configuration for sulfur is 1s 2 2s 2 2p 6 3 s 2 3p 4 and can be represented using the orbital diagram below. Exercises . Write the electron configuration for phosphorus and draw the orbital diagram.
Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...


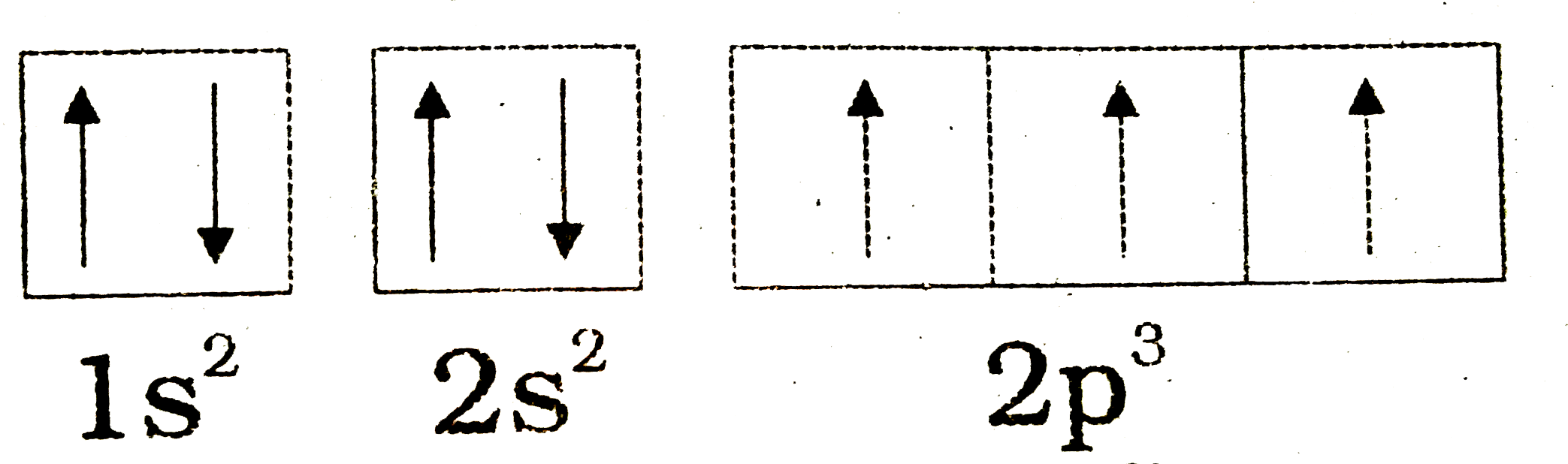

























Comments
Post a Comment