39 bromine bohr diagram
Chemistry B Flashcards - Quizlet 1. the number of protons plus neutrons in the nucleus 2 electrons 2. negative subatomic particles; symbolized by e- 4 isotope 3. atom with a net charge caused by unequal numbers of electrons and protons Bohr Diagram - All about bromine Who discovered Bromine? The atomic mass and atomic number of bromine; Physical properties of Bromine; Bromine's location in nature; Melting and boiling points of Bromine; Other elements in the group of Bromine; Type of element; Compounds it is used in ; Uses for Bromine; Unique info for bromine; location ; Bohr Diagram; lewis dot; Aufbou; Sources
File:35 bromine (Br) enhanced Bohr model.png ... June 14, 2016 - If you are browsing Commons for the first time, you may want to start with Featured pictures, Quality images, Valued images or Featured media. You can also see some work created by our highly skilled contributors in Meet our photographers and Meet our illustrators.

Bromine bohr diagram
Answered: 23) Translate the structure below to a… | bartleby Solution for 23) Translate the structure below to a Fischer projection and assign absolute configurations to the chiral carbons. 0,0, H 9 H. OH но HOH CO2H C-oH… Bohr-Rutherford Diagram for Bromine (Br) - YouTube Bromine has 18 electrons in its third shell because it is past Zinc on the periodic table. Then, you go back to the fourth shell and put 5 extra electrons in... Bohr Model of all Elements (Diagrams + Chart Inside) Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of ...
Bromine bohr diagram. Important Questions for CBSE Class 9 Science Chapter 4 ... 43. Describe Bohr’s model of the atom. Ans: There are some drawbacks in Rutherford’s atomic model. So to overcome this and to explain the structure of atoms in detail Neil Bohr in 1912 proposed a model of atoms. The postulates of Bohr’s model are given below: An electron revolves around the nucleus in the orbit of an atom with fixed energy. Bohr-Rutherford Diagram of Krypton (Kr) - YouTube Krypton has a full octet, 8 electrons, in its outer shell. It has an absolutely full third energy level (18 electrons), and then 8 in its fourth shell. Its e... How to Draw the Bohr-Rutherford Diagram for Calcium - YouTube Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: Chem4Kids.com: Bromine: Orbital and Bonding Info Chem4Kids.com! Bromine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
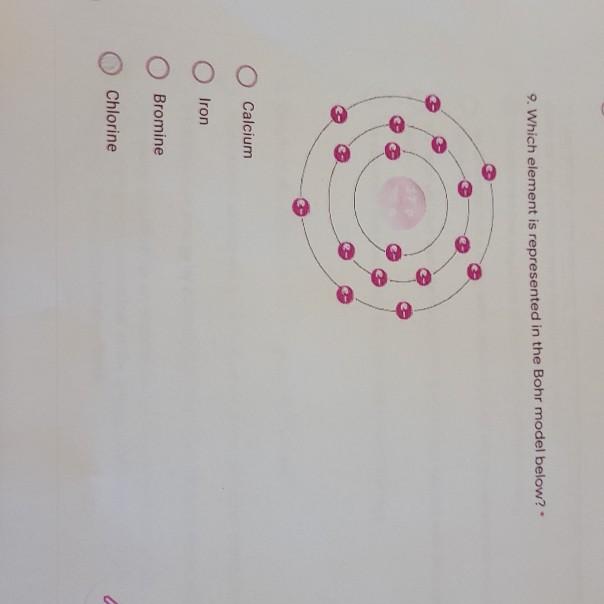
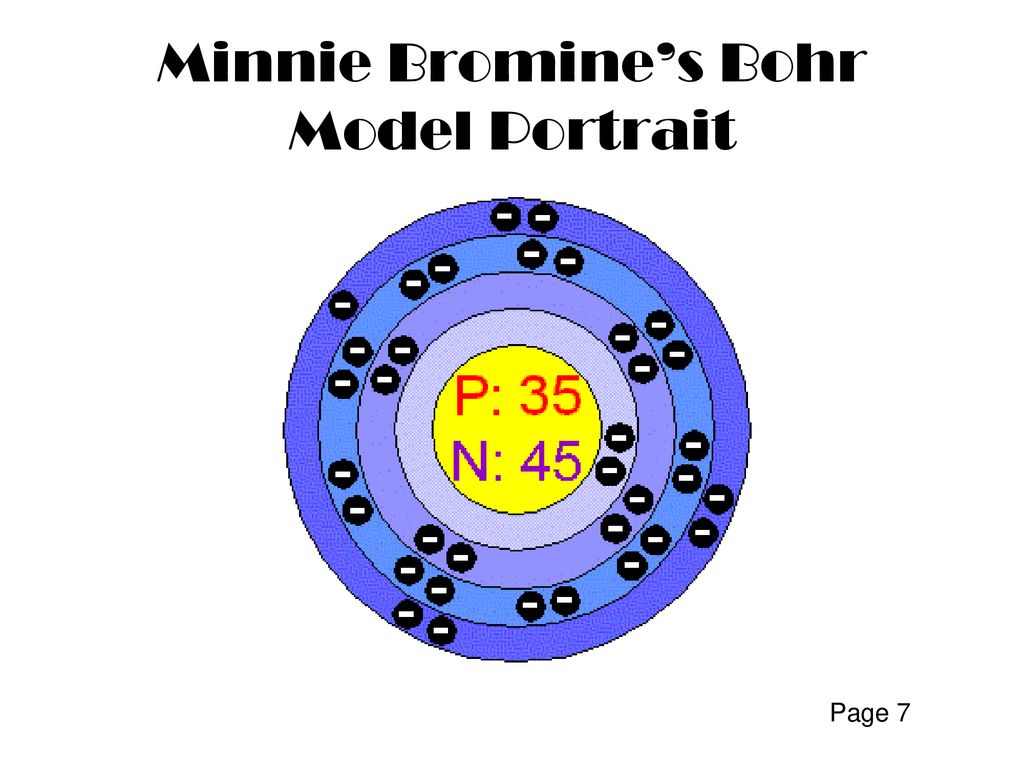
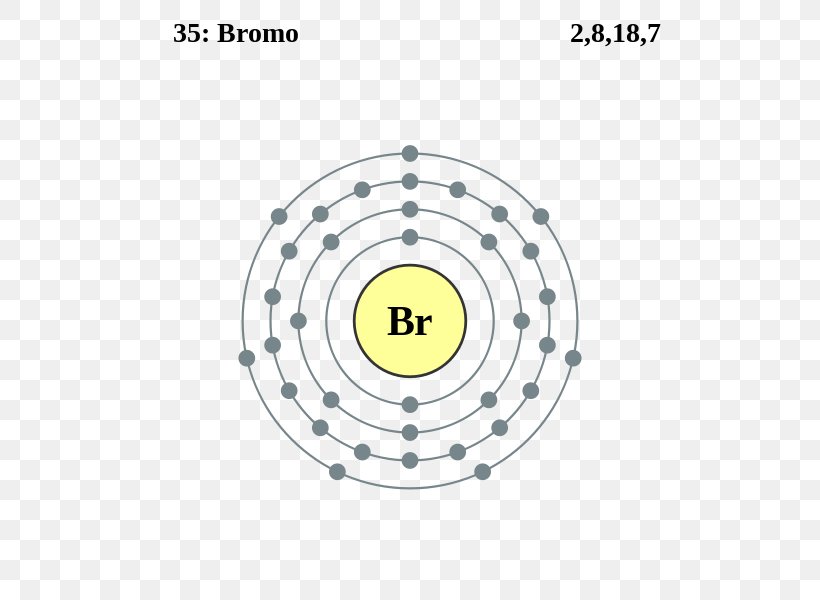
How to Do Bohr Diagrams | Sciencing April 25, 2017 - A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Atomic Diagram of Br - YouTube Atomic Diagram of Bromine - How many electrons are in each shell around the Bromine nucleus Famous scientists A-Z - Q-files - Search • Read • Discover Bohr had the idea that electrons travel in clearly defined orbits. In order to jump between them, the electrons need to either give off or absorb specific amounts (quanta) of energy. This model is the one scientists accept today. Bohr’s work earned him the Nobel Prize in Physics in 1922. During World War II, he fled Denmark. Show The Orbital-filling Diagram For Br (bromine) The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. The four concentric circles represent the four energy levels in which the electrons of a bromine atom sit.
Bromine(Br) electron configuration and orbital diagram Bromine (Br) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Br Bromine Element Information: Facts, Properties, Trends, uses ... November 25, 2020 - Br Bromine Element information, facts. Bromine properties, uses and trends | Periodic Table of the Elements - complete information about the bromine element - Facts, atomic mass, melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal ... Bromine, atomic structure - Stock Image - C018/3716 - Science Photo ... Bromine (Br). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of bromine-80 (atomic number: 35), an isotope of this element. Show The Orbital Filling Diagram For Br Bromine What Is the Orbital Diagram for Bromine? The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron.
7.3 Lewis Symbols and Structures – Chemistry 1.1 Chemistry in Context · 1.2 Phases and Classification of Matter
Atomic Structure Worksheet - Washoe County School District Bohr Diagram. Be. Bohr Diagram. Cl. Bohr Diagram Physical Science Name/Per/Due date_____ Valence Electron Practice. Directions: Give the total number of electrons and the number of valence electrons for each element listed below. Hydrogen 2. Lithium. Beryllium 4. Carbon. Fluorine 6. Neon. Magnesium 8. Chlorine. Arsenic 10. Krypton. Barium 12.
Bohr-Rutherford Diagram of Scandium (Sc) - YouTube Scandium is the first EXCEPTION to the 2-8-8-2 rule that you probably learned for Bohr-Rutherford Diagrams.All of the electrons from 21 to 30 will go into th...
How to Draw the Lewis Dot Structure for NaBr ... - YouTube A step-by-step explanation of how to draw the NaBr Lewis Dot Structure.For NaBr we have an ionic compound and we need to take that into account when we draw ...
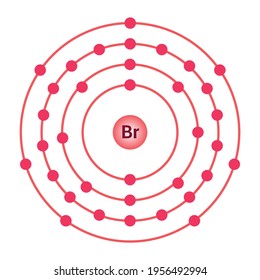
Bromine (Br) - Periodic Table (Element Information & More) Electrons arrangement in Bromine or Bohr model of Bromine: 2, 8, 18, 7: Electronic configuration of Bromine [Ar] 3d 10 4s 2 4p 5: Atomic radius of Bromine: 183 picometers (van der Waals radius) Valence electrons in Bromine: 5: 1st Ionization energy of Bromine: 13.61 eV: Electronegativity of Bromine: 2.96 (Pauling scale) Crystal structure of ...
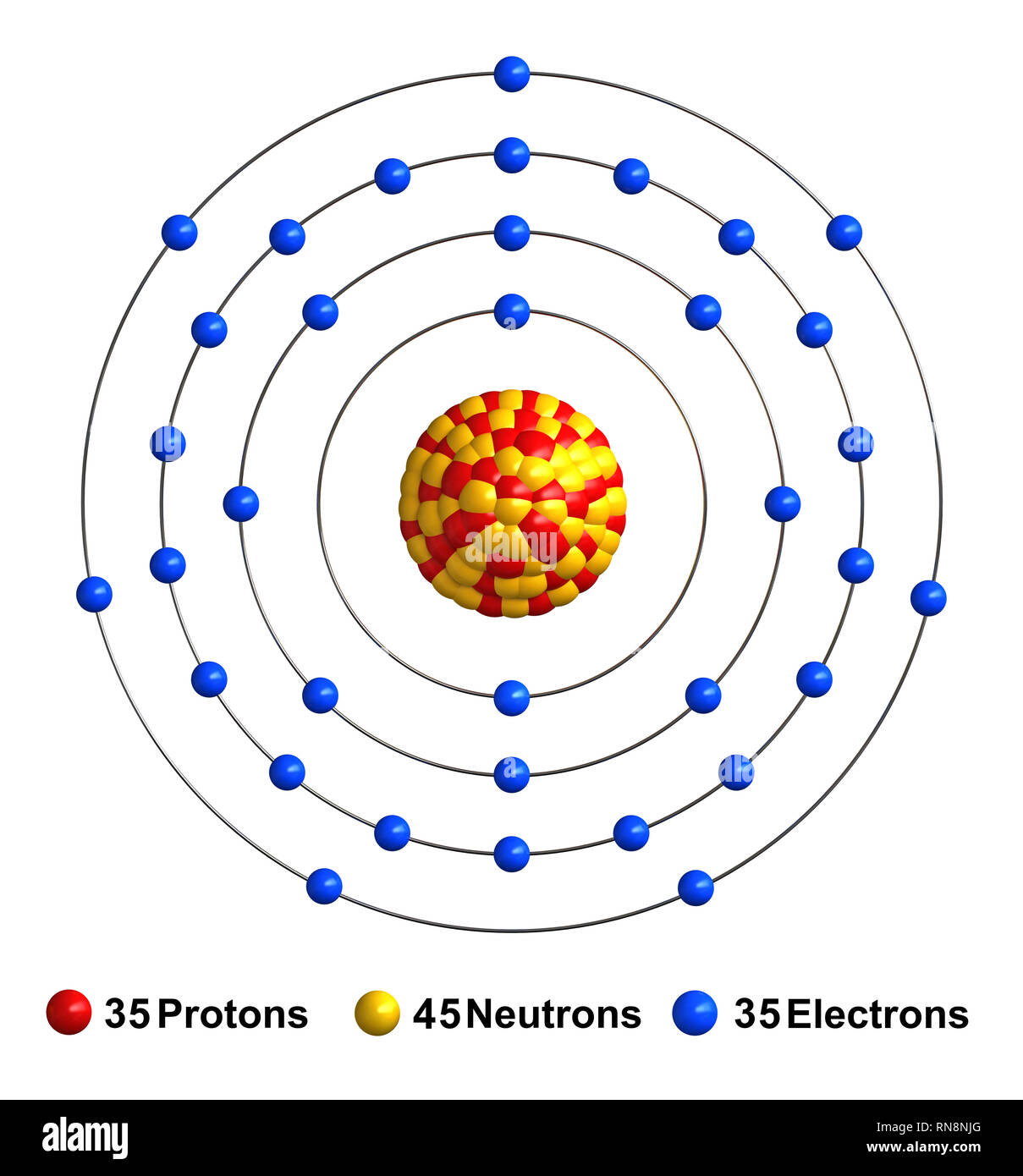
Bromine (Br) - Chemical Elements.com Name: Bromine Symbol: Br Atomic Number: 35 Atomic Mass: 79.904 amu Melting Point:-7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35 Number of Neutrons: 45 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.119 g/cm 3 Color: Red Atomic Structure
Sodium(Na) electron configuration and orbital diagram Sodium(Na) is the 11th element in the periodic table and its symbol is ‘Na’. This article gives an idea about the electron configuration of sodium and orbital diagram, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.
Bromine Bohr Diagram A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. already exists as an alternate of this question.
How to draw Bohr diagram for Bromine (Br) atom ... Bohr's diagram of Bromine has four electron shells (K, L, M, N), the inner shell is K-shell and the outermost shell is N-shell. Hence, the electrons found in the N-shell of the Bromine atom are its valence electrons because it is the outermost shell that also called the valence shell.
Show The Orbital-filling Diagram For Br (bromine) Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top . Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full version of this is.
What is the bohr rutherford diagram for bromine All Topics Topic Science Chemistry » What is the bohr rutherford diagram for bromine krishna100 Posts: 1, Reputation: 1. New Member : Apr 9, 2008, 08:13 PM What is the bohr rutherford diagram for bromine. Please show the bohr rutherford diagram for bromine ...
Bromine, atomic structure - Stock Image - C013/1582 ... Bromine (Br). Diagram of the nuclear composition and electron configuration of an atom of bromine-79 (atomic number: 35), the most common isotope of this element. The nucleus consists of 35 protons (red) and 44 neutrons (blue). 35 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
Bromine on the Periodic Table - Weebly Physical and Atomic Data Of Bromine! Bohr Diagram, Compounds that contain the element, uses for the element; Unique Information on Bromine and citations! The element Bromine is part of the halogen group of elements and is a heavy nonmetallic liquid. This nonmetallic liquid is located at group 17 and period 4 on the periodic table of elements.
Bromine Bohr Diagram - schematron.org In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of the Solar System. Transcript of Atomic Model of Bromine.
What is Bromine's Bohr Diagram? - Answers The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. What is the Bohr-Rutherford diagram of carbon?
Chemical Elements.com - Bromine (Br) Name: Bromine Symbol: Br Atomic Number: 35. Atomic Mass: 79.904 amu ... [Bohr Model of Bromine], Number of Energy Levels: 4. First Energy Level: 2
2. Bohr Models, Ionic and Covalent Bonding - YouTube Bohr models showing electron arrangement and how valence electrons are involved in ionic and covalent bonding.
PDF How to Draw Bohr Diagrams Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Bromine Atomic Structure - Br Bromine Atomic Structure · Graphic courtesy of ChemicalElements.com
Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts August 16, 2020 - Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, …
Atom Diagrams: Electron Configurations of the Elements Nov 05, 2019 · For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
Bohr Model of all Elements (Diagrams + Chart Inside) Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of ...
Bohr-Rutherford Diagram for Bromine (Br) - YouTube Bromine has 18 electrons in its third shell because it is past Zinc on the periodic table. Then, you go back to the fourth shell and put 5 extra electrons in...
Answered: 23) Translate the structure below to a… | bartleby Solution for 23) Translate the structure below to a Fischer projection and assign absolute configurations to the chiral carbons. 0,0, H 9 H. OH но HOH CO2H C-oH…



























Comments
Post a Comment