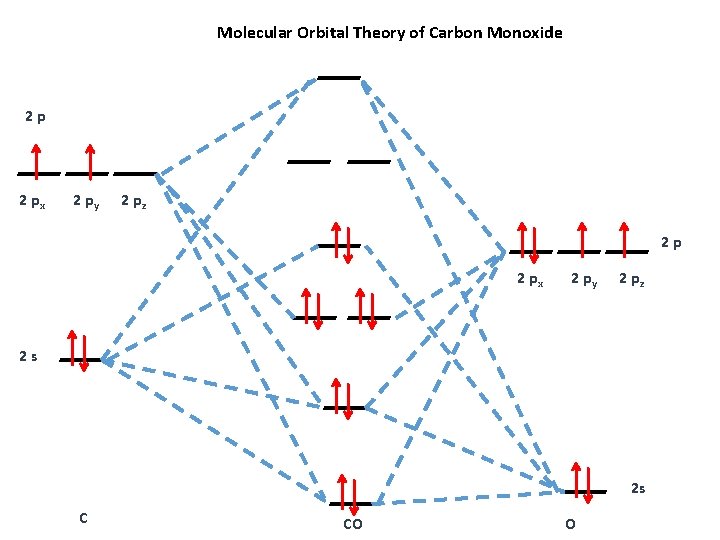
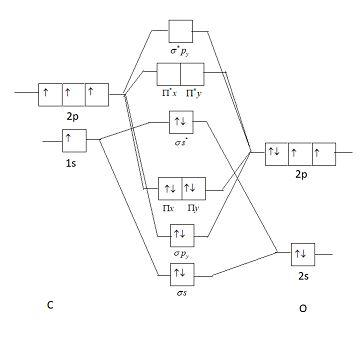
39 molecular orbital diagram for co
PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently lower in energy than the valence Does your MO diagram agree with this expectation? Determine the primary MOs that determine the bond order. Molecular orbital diagram of CO. | Download Scientific Diagram ... simple molecular orbital (MO) diagram for CO is shown below (Figure 1). The highest occupied molecular orbital (HOMO) is indicated by ... et al. studied cyclopentadienylmolybdenum carbonyls Cp2Mo2(CO)n (Cp = η 5 -C5H5; n = 6−1) (Figure 12) by density functional theory and predicted...
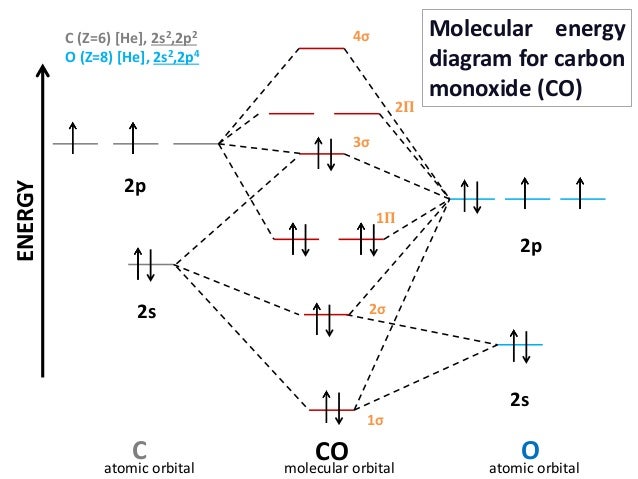
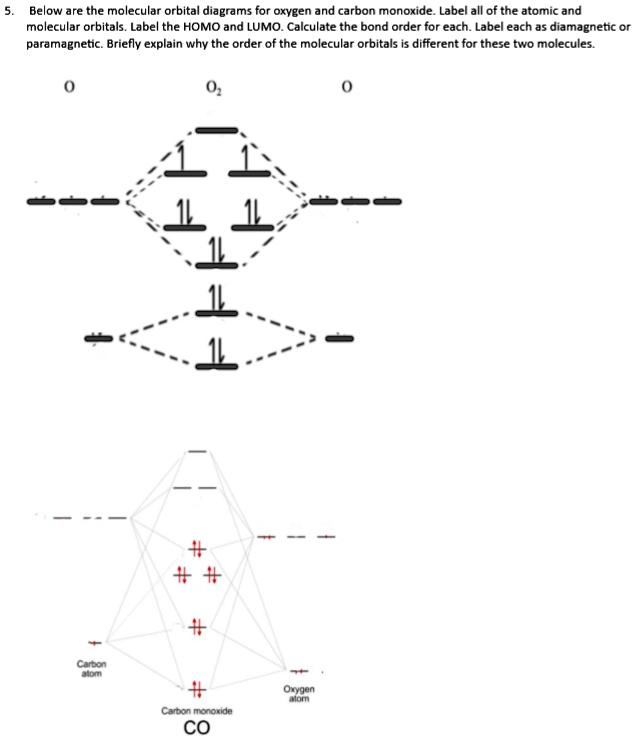
CHAPTER 5: MOLECULAR ORBITALS Li2 has a bond order of 1.0 (two electrons in a σ bonding orbital; see ... NO bond order = 2.5 (one more (antibonding) electron than CO).29 pages
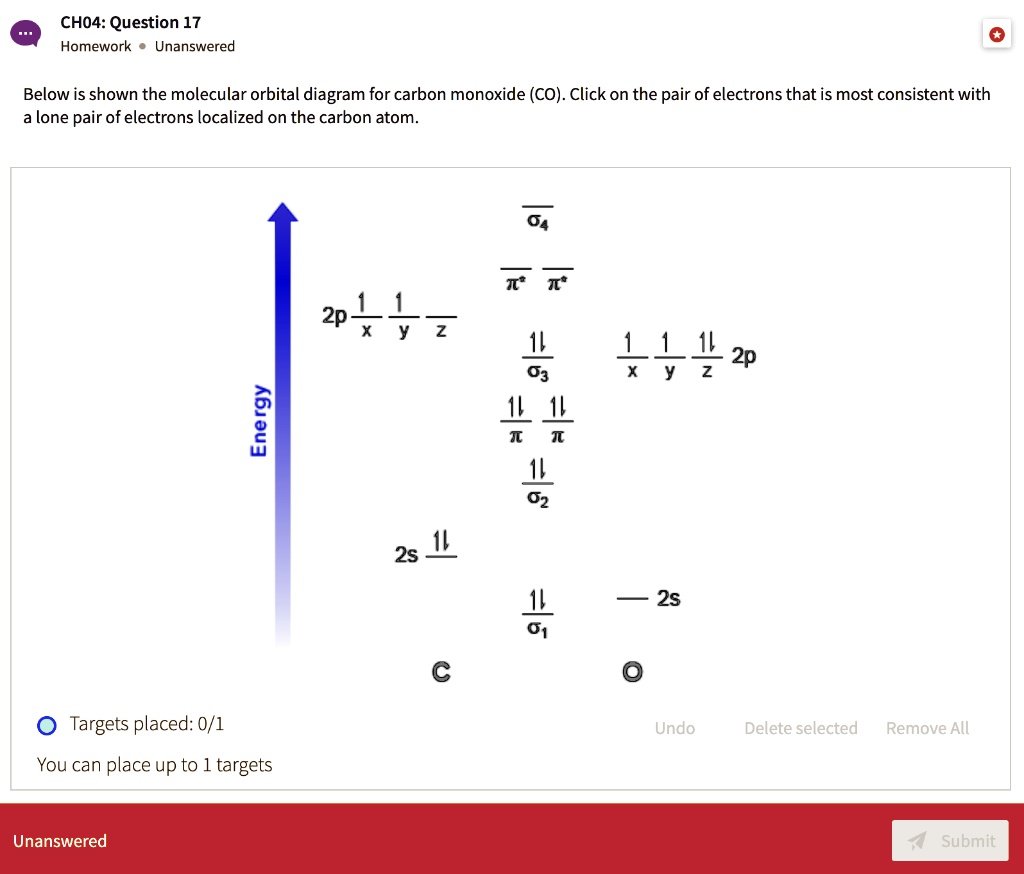
Molecular orbital diagram for co
Introduction to Molecular Orbital Theory The upper molecular orbital has a node in the electronic wave function and the electron density is low between the two positively charged nuclei. This molecule has ten electrons. The atomic orbitals combine to produce the following molecular orbital diagram: Here the 2pg orbital is occupied by... Jmol Molecular Models Showing Orbitals for CO Molecular Orbitals for CO. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital in the energy level correlation diagram shown. The results displayed may be switched between those from a low level of calculation and those from a high level. Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
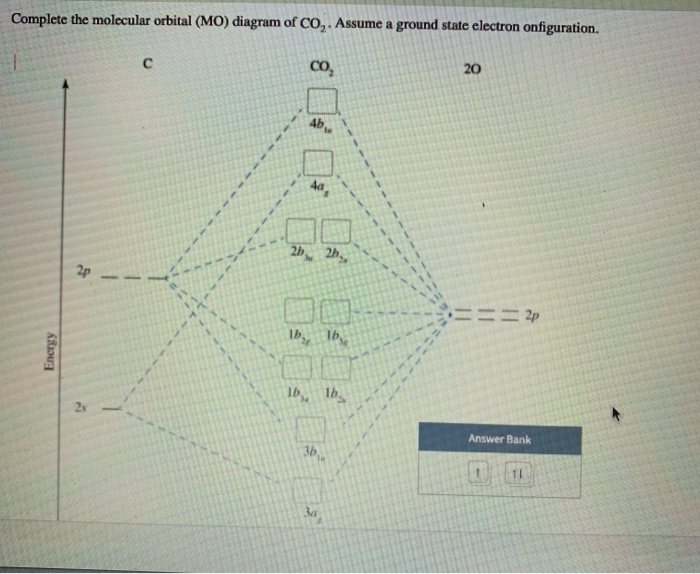
Molecular orbital diagram for co. Solved A molecular orbital diagram for CO2 is shown. | Chegg.com Transcribed image text: Molecular orbital diagram for CO2 σ* JT* 0% 2p, 2 p, 2 p lone pair 2 s lone pair 2 S carbon oxygen. Molecular orbital theory. Heteronuclear diatomics. CO - YouTube 12-12 This video describes the molecular orbital theory diagram of CO, placing emphasis on how MO theory differs for homo and heteronuclear diatomics. Molecular Orbital diagram of Carbon monoxide molecule (CO) Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... MO approach to more complex molecules and CO bonding in transition metals complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
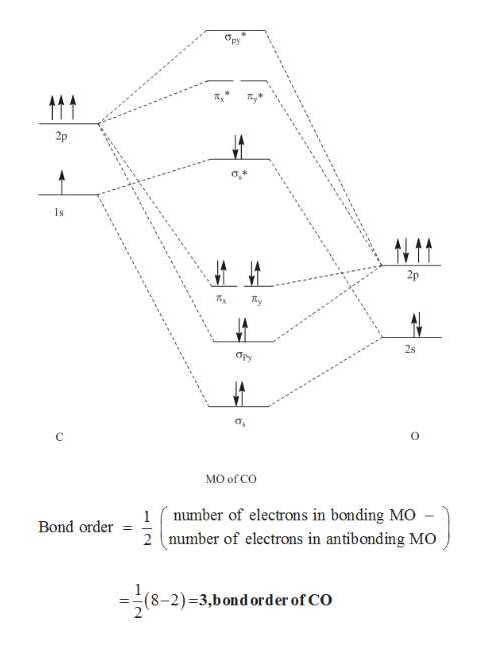
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. PDF Chapter 5 | 5.2.2 Orbital Mixing The MO diagram of CO helps explain its reaction chemistry with transition metals, which is different than that predicted by electronegativity considerations that would suggest However, these orbital classifications are consistent with a threefold bond order for CO. 138 Chapter 5 | Molecular Orbitals. MOLECULAR ORBITAL ENERGY LEVEL DIAGRAM OF CO Page 1. •. MOLECULAR ORBITAL ENERGY LEVEL DIAGRAM OF CO. Thursday, 14 January 2021. 10:49 PM.1 page What is the Molecular orbital theory diagram for co? - Answers A full molecular orbital treatment shows that there is a pair of electrons on the carbon that can be donated to the metal forming a sigma bond; There are filled d orbitals on the metal that "back donate" into empty anti-bonding molecular orbitals on the CO. This is a push me pull you synergistic effect.
PDF Orbitals and molecular representation orbital... Construct the molecular orbital diagram for dichlorine. Draw the bonding orbitals for: H2O NH3 CO CO2. Orbitals and molecular representation representations of molecules. An introduction to molecular orbital theory The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive... Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. A Because sodium has a [Ne]3s1 electron configuration, the molecular orbital energy-level diagram is qualitatively identical to the diagram for the interaction of two 1s... Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
What is the molecular orbital energy diagram of CO? - Quora 7 Jan 2017 — The geometry of this molecule about the C atom is trigonal planar, with 120 degrees separating each of the 3 sigma bonds. The carbon atom therefore hybridises ...
PDF PowerPoint Presentation Simplified MO energy level diagram for Cr(CO)6. Note the empty π* orbitals. Only three are involved in overlap with metal d orbitals. Figure 3-1 Molecular orbitals of Cr(CO)6 (Only interactions between Ligand (σ- and π*) orbitals and metal d-orbitals are shown.)
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are In heteronuclear diatomic molecules, atomic orbitals only mix when the electronegativity values are similar. In carbon monoxide (CO), the oxygen 2s...
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
PDF Microsoft Word - Chapter 1_6_SY.doc The molecular orbital diagram for dihelium (He2) is the same as that of hydrogen, with the addition of two more electrons (Figure 9.37). Chapter 9. The molecular orbital diagram for the second row homonuclear. Chapter 9. Theories of Chemical Bonding.
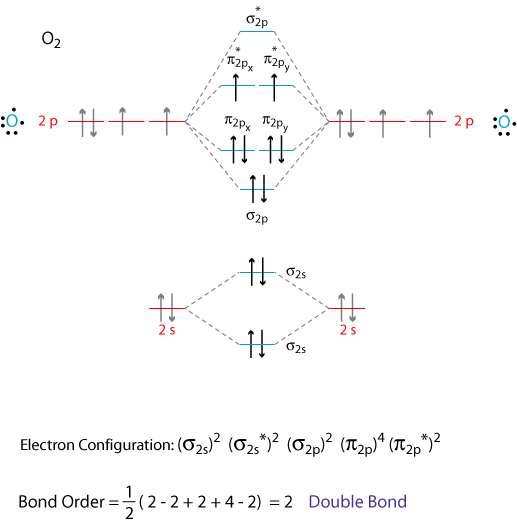
Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...
Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: It is used to define the bonding in molecules which cannot be explained with the help of Valence Bond Theory. Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals.
8.4 Molecular Orbital Theory - Chemistry The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
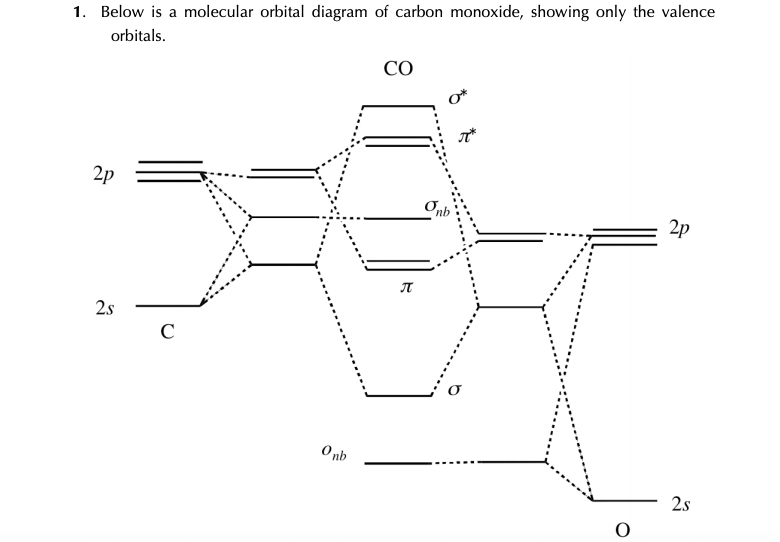
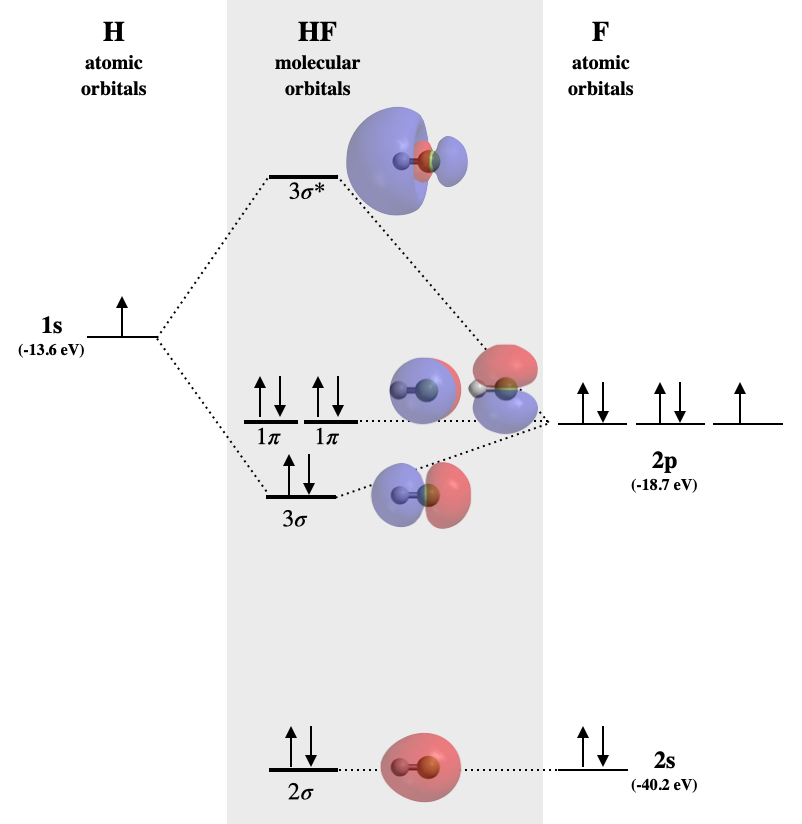
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Accordingly, a molecular orbital diagram such as Figure 9-5 is inappropriate for heteronuclear diatomic molecules. If the two elements are similar (as in NO or Construction of the MO diagram for CO is a complex case, beyond the coverage in this textbook. Figure 9-9 MO energy level diagram for...
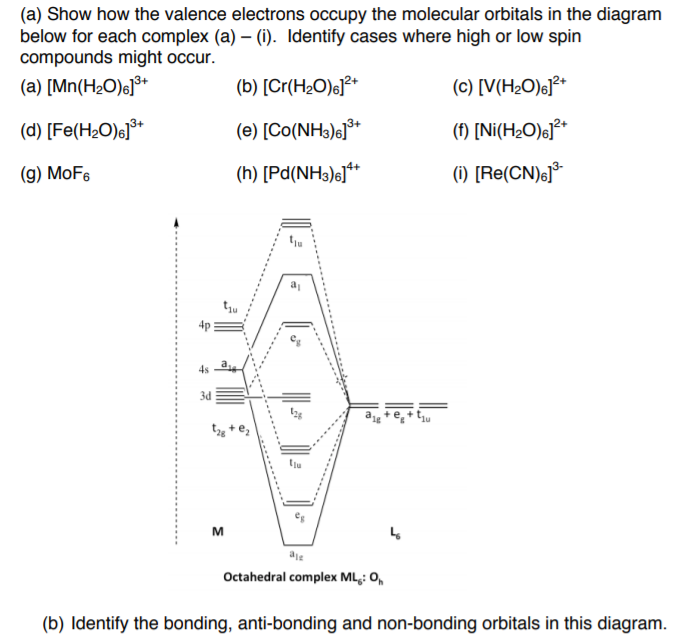
PDF Lecture 1 Octahedral ML6 molecular orbital energy diagram. Lecture 3: p-acceptor ligands and synergic bonding. p-accepting orbitals. The major interaction is s-donation from the CO 5s orbital to the metal giving weak M-CO bonding. The CO stretching frequency is > free CO mainly due to electrostatic...
Molecular Orbitals: Molecular Orbital Theory | SparkNotes Molecular orbital theory posits the notion that electrons in molecules likewise exist in different orbitals that give the probability of finding the electron at Notice that the orbitals of the separated atoms are written on either side of the diagram as horizontal lines at heights denoting their relative energies.
Molecular Orbital diagram for CO - Ultraviolet and Visible... | Coursera Here we have a molecular orbital diagram for the CO molecule. So when you're drawing on a global diagram like this, you have to draw it, it should be schematically shown lower energy than the carbon.
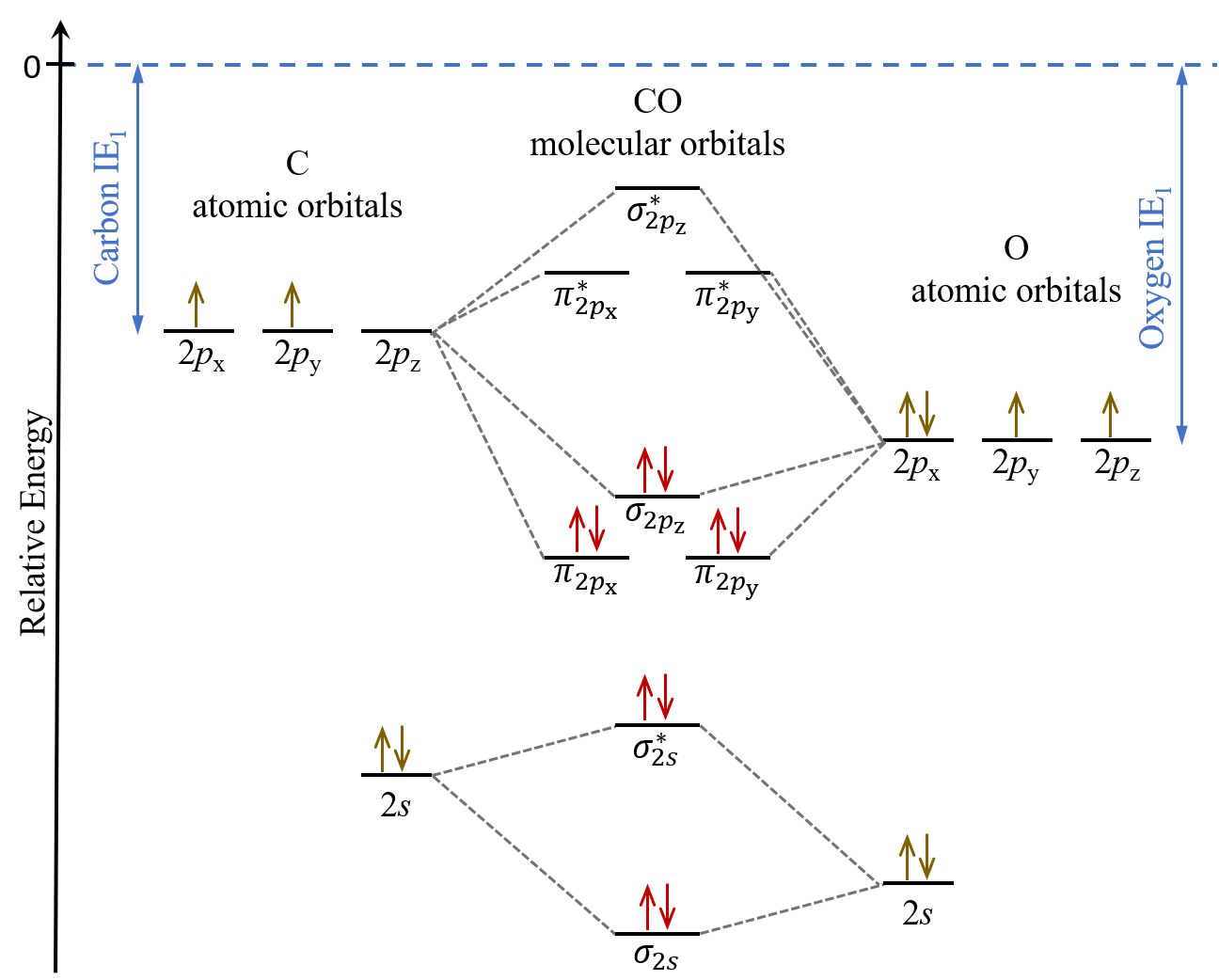
What is the molecular orbital energy diagram of CO? - Quora A molecular orbital diagram , or MO diagram , is a qualitative descriptive tool explaining chemical bonding. The discrepancy in energies allows the π2px & π2py bonding molecular orbitals to sink lower in energy than the " σ*2s MO" in the MO diagram of CO.
Energy level diagram for Molecular orbitals - Chemical Bonding and... 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e
How can I read molecular orbital diagram? | Socratic Let's analyze a molecular orbital (MO) diagram for CO. academics.wellesley.edu. Points to Note: Individual atomic orbitals (AOs) are on the far left (C) and far right (O) of the diagram. These AOs overlap to produce the MOs of CO in the middle.
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
Jmol Molecular Models Showing Orbitals for CO Molecular Orbitals for CO. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital in the energy level correlation diagram shown. The results displayed may be switched between those from a low level of calculation and those from a high level.
Introduction to Molecular Orbital Theory The upper molecular orbital has a node in the electronic wave function and the electron density is low between the two positively charged nuclei. This molecule has ten electrons. The atomic orbitals combine to produce the following molecular orbital diagram: Here the 2pg orbital is occupied by...













![Exchange coupling through diamagnetic [Fe(CO) 4 ] 2 ...](https://pubs.rsc.org/image/article/2011/DT/c0dt01221a/c0dt01221a-f2.gif)






Comments
Post a Comment