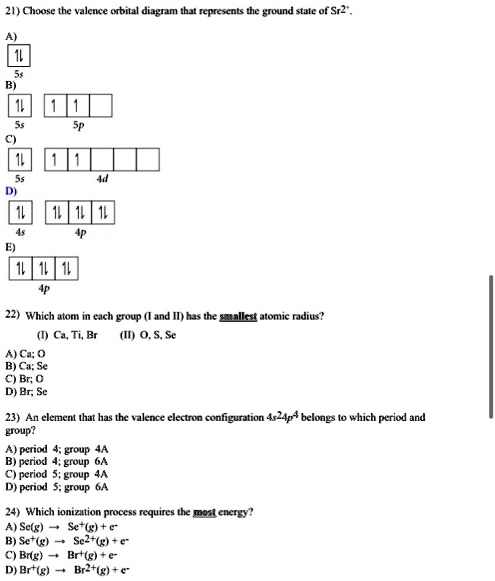
39 orbital diagram for br
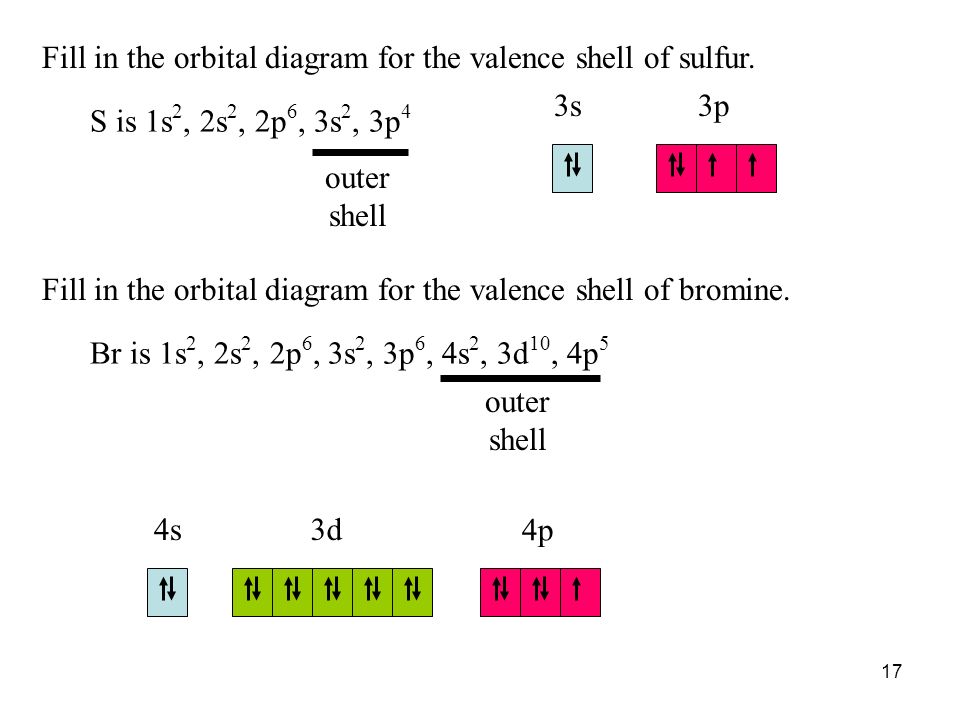
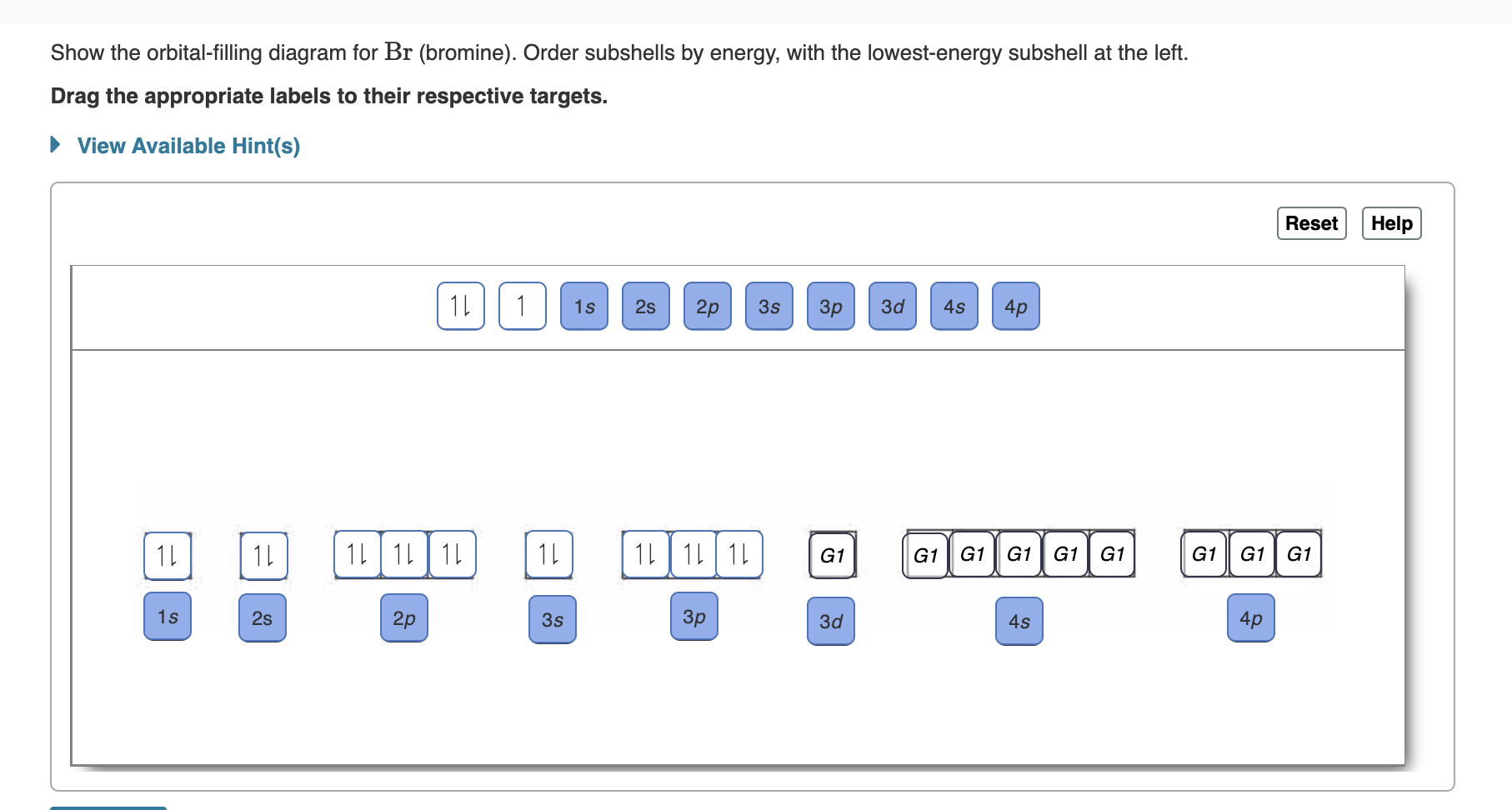
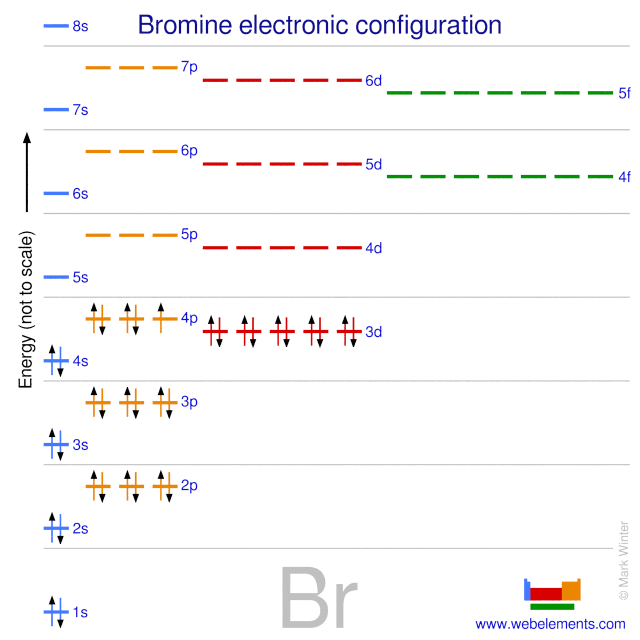
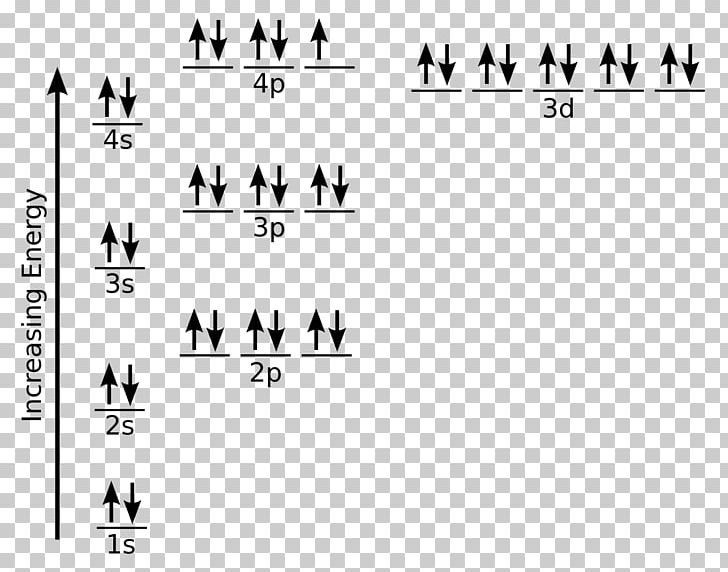
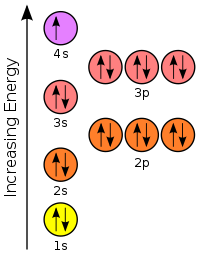
Bromine(Br) electron configuration and orbital diagram Bromine (Br) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund’s principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Orbital Filling Diagram For Bromine Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top .Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top.
Phosphorus(P) electron configuration and orbital diagram Orbital diagram for phosphorus (P) Phosphorus(P) excited state electron configuration. Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub ...

Orbital diagram for br
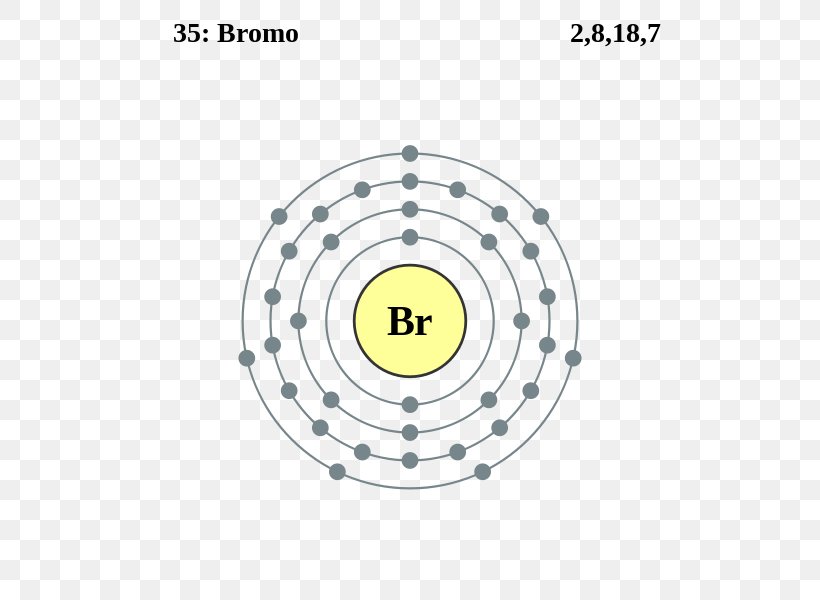
Show The Orbital-filling Diagram For Br (bromine) The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. The four concentric circles represent the four energy levels in which the electrons of a bromine atom sit. Molecular orbital diagrams of Cl2, H2O, and Br2 ... Download scientific diagram | Molecular orbital diagrams of Cl2, H2O, and Br2. from publication: Theoretical Study of the Potential Energy Surfaces of the Van Der Waals H 2 O−X 2 + (X = Cl or Br ... Solved Which of the following is the correct ground state ... Question: Which of the following is the correct ground state orbital diagram for the ion Br" (Z = 35)? A B D E Ener 22 I O none of these OD ОЕ ОА OB Ос . This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer.
Orbital diagram for br. Bromine Orbital Diagram - Periodic Table Bromine Orbital Diagram. How Can We Find A Electron Configuration For Bromine (Br) May 9, 2021 by Sneha Leave a Comment. Are you seeking the How Can We Find A Electron Configuration for Bromine. Do you know bromine is a chemical element that you can found in the periodic table? The atomic weight of the bromine is 79.904, and the atomic number ... Orbital Diagram of Br part II - YouTube Orbital Diagram of Br What is the orbital diagram for bromine? - Answers What is Bromine's Bohr Diagram? m What is an orbital diagram? An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is... Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram of Niobium (Nb) 42: Orbital diagram of Molybdenum (Mo) 43:
Show The Orbital Filling Diagram For Br Bromine stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy.the orbital doagram for bromine is the orbital diagram for bromine is the orbital diagram for bromine is mar 22, · best answer: yes; bromine (atomic number 35) has 1 less electron than the next higher inert gas, krypton (atomic number 36) … Bromine Orbital Diagram Feb 08, 2018 · The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. Bromine Electron Configuration (Br) with Orbital Diagram Feb 01, 2021 · Bromine Orbital Diagram Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. You will see that the 3d sublevel is filled before the 4p after the 4s. Br2 Lewis Structure, Molecular Geometry, Hybridization ... The MO diagram or Molecular Orbital diagram is an extension of the 3-dimensional molecular design and gives a better understanding of the structure of an atom. Molecular Diagram also reflects upon bond length, bond shape, bond energy, and the bond angle between 2 atoms. Br2 is a simple compound as it is formed by 2 atoms of the same element.
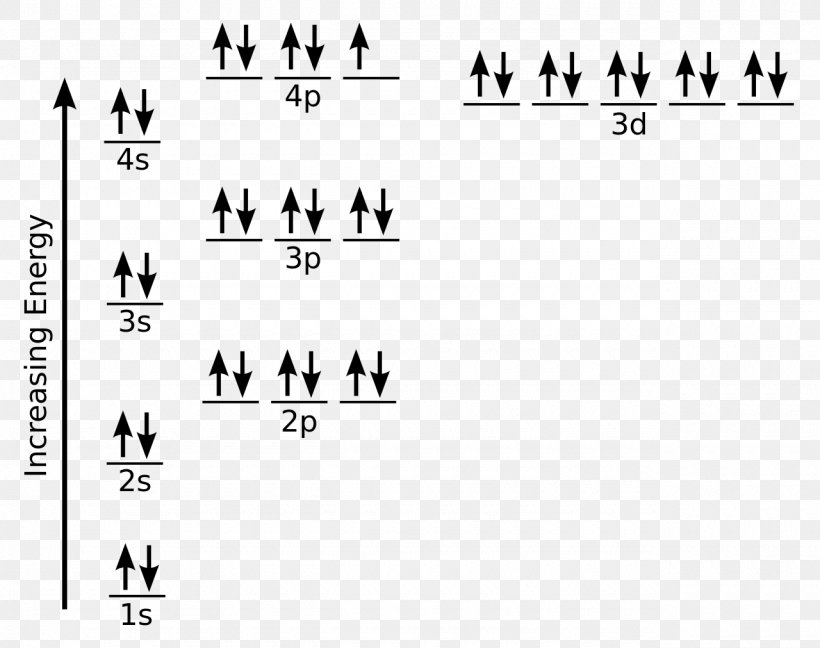
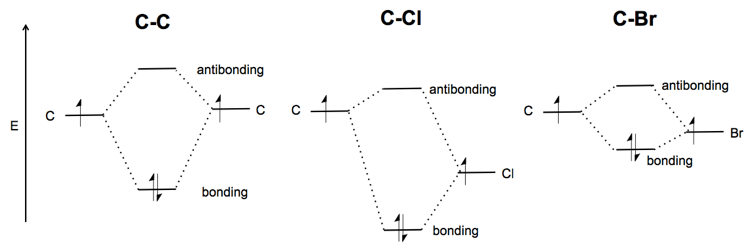
How to Do Orbital Diagrams - Sciencing The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ... Solved Give the orbital diagram of a bromine (Br) atom (Z ... Science. Chemistry. Chemistry questions and answers. Give the orbital diagram of a bromine (Br) atom (Z = 35). PDF Chapter 8 Orbital diagrams make use of a box, circle, or line for each orbital in the energy level. An arrow is used to represent an ... Kr is smaller than Br because Kr is further to the right in the same period. (d) Rb > Sr > Ca. Ca is the smallest because it has one fewer energy level. Sr is smaller Hbr Mo Diagram - Diagram World From the MO diagram we see that all four electrons in the p non-bonding orbitals come from the Brs 4p orbitals. It is utilized in the production of inorganic bromides and organic bromides in reaction with alkynes to form bromoalkenes opening epoxides and lactones as a catalyst for oxidations and in. Identify The Correct MO Energy Diagram For HBr.
Br 2+ - CHEMISTRY COMMUNITY Br has 7 valence electrons, so Br2 should have 14. Br2 2+, however, should have 2 less electrons. Br2 2+ should be paramagnetic because when you remove the 2 electrons, the 3pi-x and 3pi-y will be unpaired, like Shreyesi said. I'm pretty sure it's a typo, you could check with Lavelle. Top.
Electron configuration for Bromine (element 35). Orbital ... Bromine electron configuration. ← Electronic configurations of elements. Br (Bromine) is an element with position number 35 in the periodic table. Located in the IV period. Melting point: -7.3 ℃. Density: 3.14 g/cm 3 . Electronic configuration of the Bromine atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5.
What is the orbital notation for bromine? - Answers The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7.
PDF Orbital Diagrams Activity - LCPS 7. Each circle represents an orbital. How many orbitals are in each sublevel (s, p, d and f)? How many electrons can fit in each orbital? 8. The electron configuration for Br is: 1s 22s 22p 63s 23p 64s 23d 10 4p 5. Why do you think 4s comes before 3d? Compare the electron configuration to the diagram for Br. What do the superscripts represent?
What is the orbital diagram for tellurium? The electronic configuration of Bromine is 1s104p5 and the valence electrons are in the 4s and 4p orbitals giving Bromine 7 valence electrons. ... The atomic number of Bromine is 35, which means it has 7 electrons in its valence shell. It require one more electron to attain the noble gas configuration. How many inner electrons does BR have? 35
BrF3 Lewis Structure, Molecular Geometry, Hybridization ... Br: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 Br: [Ar] 4s2 3d10 4p5. When we are looking at the bond formation of Br with each of the fluorine atoms, the paired electrons will shift to fill up the 4d orbital. Thus we have the one s orbital, px, py, and pz orbitals and one d orbital( dxy for example)
PDF Electron Configurations and Orbital Diagrams key Electron Configurations and Orbital Diagrams KEY Draw orbital diagrams for the following elements: ... 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6
How to Write the Atomic Orbital Diagram for Bromine (Br ... To write the orbital diagram for the Bromine atom (Br) first we need to write the electron configuration for just Br. To do that we need to find the number o...
How Can We Find A Electron Configuration For Bromine (Br ... The complete Electron Configuration of the elements bromine is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁵. You can find the highest energy orbital in S and P. The 4s and 4P make the valence Electron of bromine 7. Bromine is located in the crustal rocks of the earth. You will find bromine as a byproduct of the bromine salt.
how many half filled orbitals are in a bromine atom ... 35 Electron configuration, Orbital notation and Quantum Numbers for Bromine; 36 Bohr-Rutherford Diagram for Bromine (Br) 37 Quantum Numbers, Atomic Orbitals, and Electron Configurations; 38 The bromine atom possesses 35electrons. It contains 6 electrons in 2p orbital, 6 electrons in 3p….
Bromine (Br) - ChemicalAid Bromine (Br) has an atomic mass of 35. Find out about its chemical and physical ... Orbital Diagram. Br - Bromine - Orbital Diagram - Electron Configuration ...
Bromine Orbital Diagram - Wiring Diagrams Bromine Orbital Diagram. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). the σ bonds. I've drawn the overlaps below in the MO diagrams. Each bromine would donate one 4pz electron to form a σ -bonding orbital. Answer to Draw an orbital diagram for each element: (a) magnesium; (b) aluminum; (c) bromine. wiringall.com!
Solved Which of the following is the correct ground state ... Question: Which of the following is the correct ground state orbital diagram for the ion Br" (Z = 35)? A B D E Ener 22 I O none of these OD ОЕ ОА OB Ос . This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer.
Molecular orbital diagrams of Cl2, H2O, and Br2 ... Download scientific diagram | Molecular orbital diagrams of Cl2, H2O, and Br2. from publication: Theoretical Study of the Potential Energy Surfaces of the Van Der Waals H 2 O−X 2 + (X = Cl or Br ...
Show The Orbital-filling Diagram For Br (bromine) The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. The four concentric circles represent the four energy levels in which the electrons of a bromine atom sit.




















Comments
Post a Comment