43 co orbital diagram
Co2+ Orbital Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. The valence electron configuration of "O" is ["He"] 2s^2 2p^4. What is the molecular orbital energy diagram of CO? - Quora The geometry of this molecule about the C atom is trigonal planar, with 120 degrees separating each of the 3 sigma bonds. The carbon atom therefore hybridises ...4 answers · 29 votes: First let us know what molecular orbital diagram is: A molecular orbital diagram, or MO diagram, ...
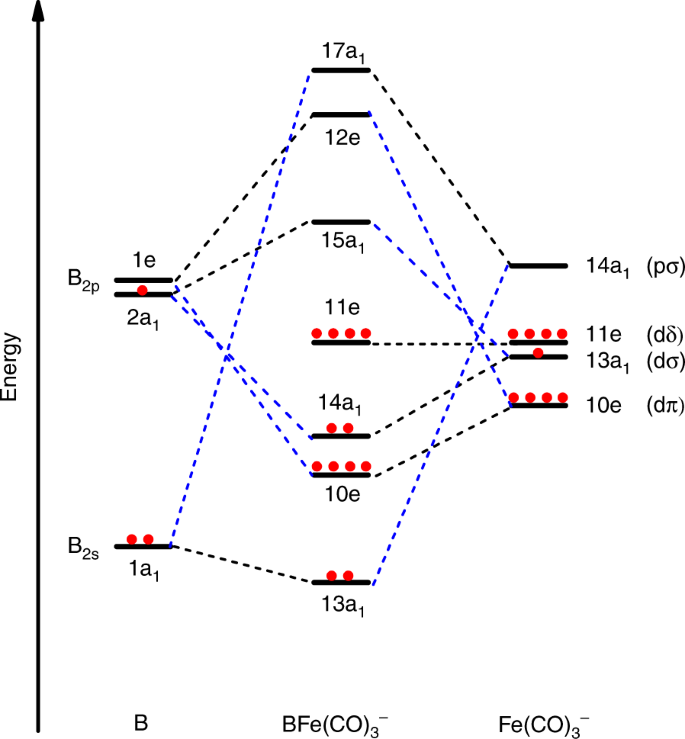
Molecular orbitals diagrams of [Co(NH3)6]3+ Molecular orbitals diagrams of [Co (NH3)6]3+. 1. M. O. diagram for [Co (NH3)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa. 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Co3+→ [Ar] 3d6, 4s0 6e- Ligand group (NH3) orbitals 6 x 2 = 12 e- σ [Co (NH3)6]3 ...
Co orbital diagram
What is the electron configuration of "Co"^"3+"? | Socratic The electron configuration of "Co"^(3+) is ["Ar"] 4s 3d^5. "Co" is in Period 4 of the Periodic Table, and "Ar" is the preceding noble gas. Cobalt is also in Group 9, so it must have 9 valence electrons. The valence shell configuration is therefore 4s^2 3d^7, and the core notation is bb"Co": ["Ar"] 4s^2 3d^7 When a transition metal forms an ion, the s electrons are removed before the d electrons. inorganic chemistry - Molecular orbital diagram of CO and ... Molecular orbital diagram of CO and charge localisation. Ask Question Asked 1 year, 9 months ago. Active 1 year, 9 months ago. Viewed 138 times 4 1 $\begingroup$ MO diagram of CO. My question concerns the interpretation of the Molecular Orbital of CO. I think I find it clear how you build it but I have some concerns about how you rationalize it. How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
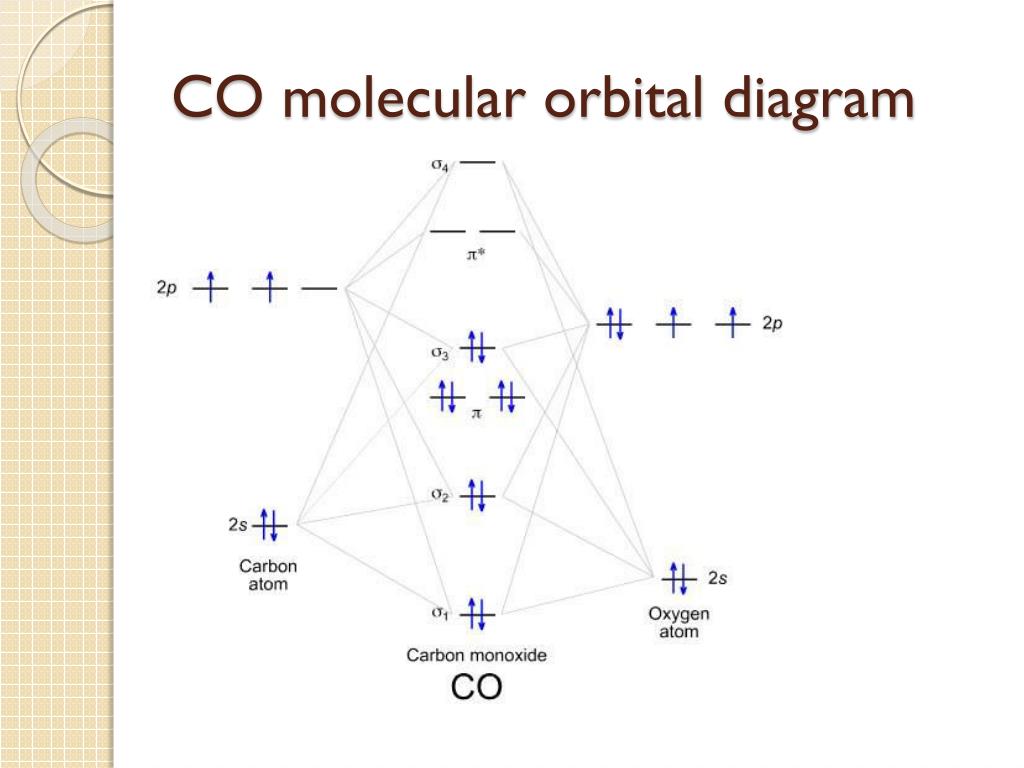
Co orbital diagram. Tanabe–Sugano diagram - Wikipedia Unnecessary diagrams: d 1, d 9 and d 10 d 1. There is no electron repulsion in a d 1 complex, and the single electron resides in the t 2g orbital ground state. A d 1 octahedral metal complex, such as [Ti(H 2 O) 6] 3+, shows a single absorption band in a UV-vis experiment. The term symbol for d 1 is 2 D, which splits into the 2 T 2g and 2 E g states. The t 2g orbital set holds the single ... Molecular Structure & Bonding - Chemistry The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond. Draw MO diagram of CO and calculate its bond order ... Draw MO diagram of CO and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 17, 2020 by Maisa (45.8k points) selected Dec 18, 2020 by Panna01 . Best answer. 1. Electronic configuration of C atom: 1s 2 2s 2 2p 2. ... covalent bonding - single bonds - chemguide The diagram of PCl 5 (like the previous diagram of PCl 3) shows only the outer electrons. Notice that the phosphorus now has 5 pairs of electrons in the outer level - certainly not a noble gas structure. You would have been content to draw PCl 3 at …
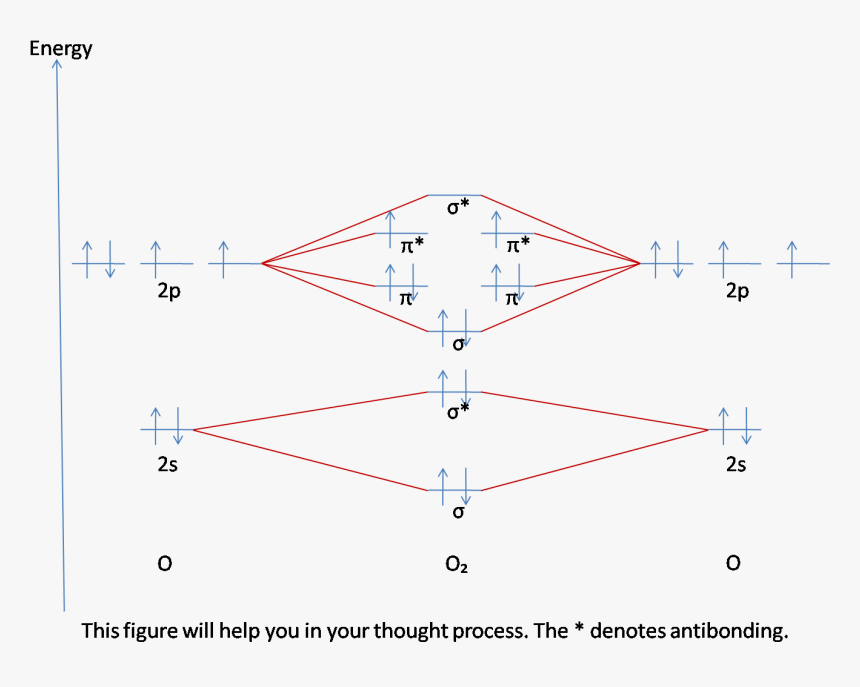
Oxygen(O) electron configuration and orbital diagram Oxygen(O) is the 8th element in the periodic table and its symbol is ‘O’. This article gives an idea about the electron configuration of oxygen and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this. How To Draw Molecular Orbital Diagram Of Co - Drawing ... Electronic configuration of co molecule is: Draw the orbital diagram for the ion co2+. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The bonding mos are the 2σ, 1πx, 1πy, and 3σ, which gives 2 +2 +2 +2 = 8 bonding electrons. The content is presented using short focussed and interactive screencast. Cobalt(Co) electron configuration and orbital diagram Atomic Orbital Diagram for Cobalt (Co) Cobalt ion(Co 2+,Co 3+)electron configuration. Ground state electron configuration of cobalt(Co) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2. The electron configuration shows that the last shell of cobalt has two electrons and the d-orbital has a total of seven electrons. In this case, the valence electrons of ... en.wikipedia.org › wiki › Orbital_hybridisationOrbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.
MOT of CO+ molecule | Tricks For Molecular Orbital Theory ... This video for molecular orbital theory (MOT) in chemical bonding for CO+ molecule is by VIKAS MALI sir . Energy diagram of COshort tricks for Chemistry eve... valenceelectrons.com › cobalt-electron-configurationCobalt(Co) electron configuration and orbital diagram Cobalt (Co) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Molecular Orbitals for Carbon Monoxide - Newcastle University Molecular Orbitals for CO. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital in the energy level correlation diagram shown The results displayed may be switched between those from a low level of calculation and those from a high level. Draw the orbital diagram for ion Co 2+. - Clutch Prep Problem: Draw the orbital diagram for ion Co 2+. FREE Expert Solution Show answer Answer: 80% (132 ratings) Sign up for free to keep watching this solution Sign up for free. 600,863. students enrolled. 97%. improved grades. 2,784. minutes of videos. Continue watching with Facebook
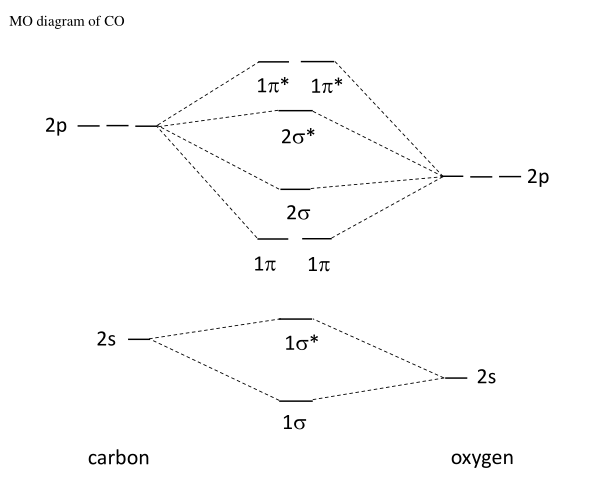
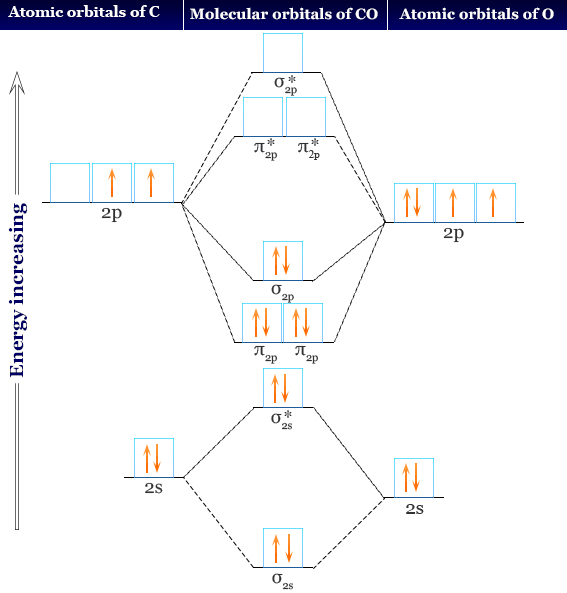
What is molecular orbital diagram of CO? - handlebar ... What is molecular orbital diagram of CO? Carbon monoxide MO diagram. Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The valence molecular orbitals in both atoms are the 2s and 2p orbitals. The molecular orbital diagram for carbon monoxide (Figure 5.3. Is there SP mixing in CO?
COHP - Crystal Orbital Hamilton Population • Theory Theory: From H 2 to Data-Storage Alloys The COHP (or COOP) concept is most easily understood by looking at the simple band structure of a "one-dimensional" solid; the following example has been stolen from a classic introduction.Imagine a linear chain of hydrogen atoms, the one-dimensionally periodic analogue of H 2 (whose molecular-orbital scheme is known …
Exploded Parts Diagrams for Zetor - MalpasOnline.co.uk Use this page to find parts you need to complete your repair/restoration of your tractor. Whether it is a vintage or a modern tractor you should be able to identify the part by make and application. Once you have found the part on a diagram click on the part number listed in …
› basicorg › bondingelectronic structure and atomic orbitals - chemguide The diagram shows a cross-section through this spherical space. 95% of the time (or any other percentage you choose), the electron will be found within a fairly easily defined region of space quite close to the nucleus. Such a region of space is called an orbital. You can think of an orbital as being the region of space in which the electron lives.
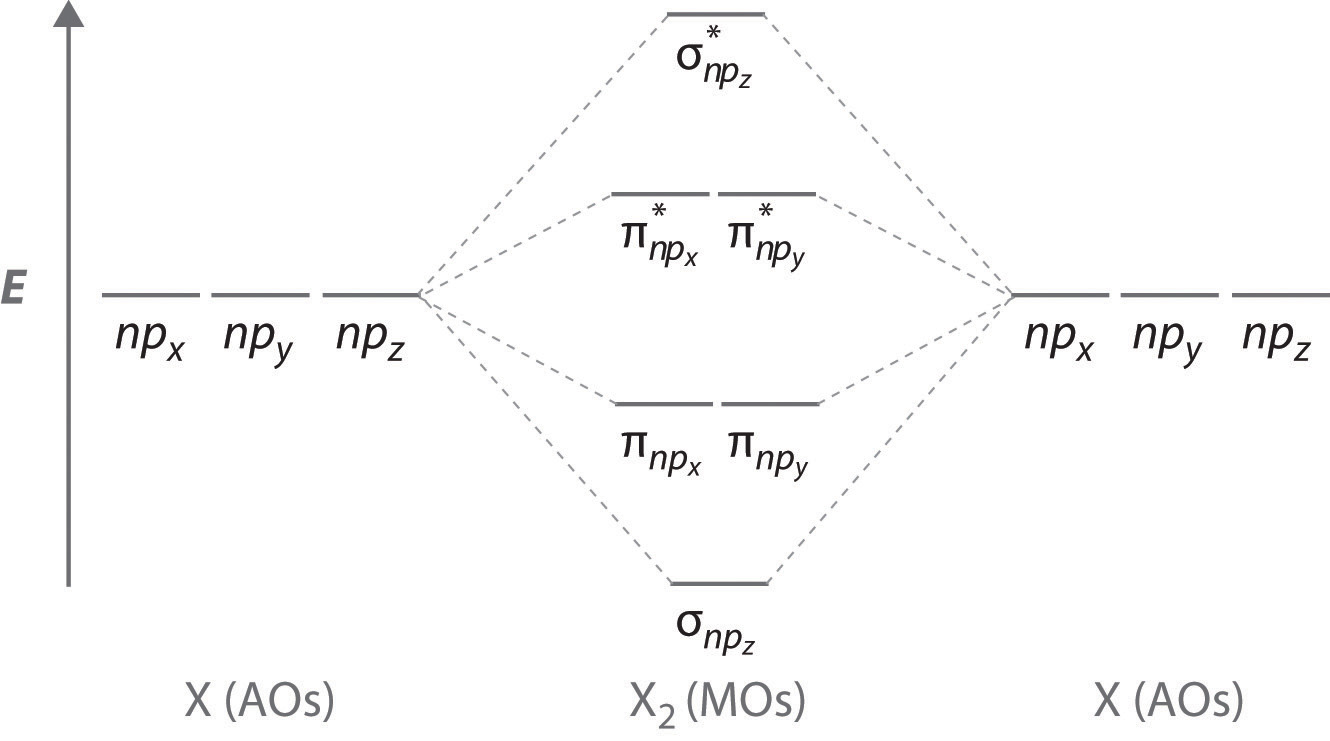
› OrbitalsORBITALS and MOLECULAR REPRESENTATION In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
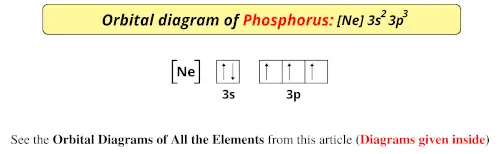
periodictableguide.com › orbital-diagram-of-allOrbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Co2+ Orbital Diagram - schematron.org Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital.
Carbon Orbital diagram, Electron configuration, and ... The orbital diagram for Carbon is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Carbon orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest two electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Carbon atom is shown below-.
en.wikipedia.org › wiki › Molecular_orbital_diagramMolecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
ssd.jpl.nasa.gov › tools › sbdb_lookupSmall-Body Database Lookup - NASA Instructions. The search form recognizes IAU numbers, designations, names, and JPL SPK-ID numbers. When searching for a particular asteroid or comet, it is best to use either the IAU number, as in 433 for asteroid “433 Eros”, or the primary designation as in 1998 SF36 for asteroid “25143 (1998 SF36)”.
Carbon Monoxide Molecular Orbital Diagram Explanation Carbon Monoxide Molecular Orbital Diagram Explanation. generic s-p valence MO diagram for carbon monoxide CO chain one can reasonably explain, that the HOMO of carbon monoxide must be of. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not.
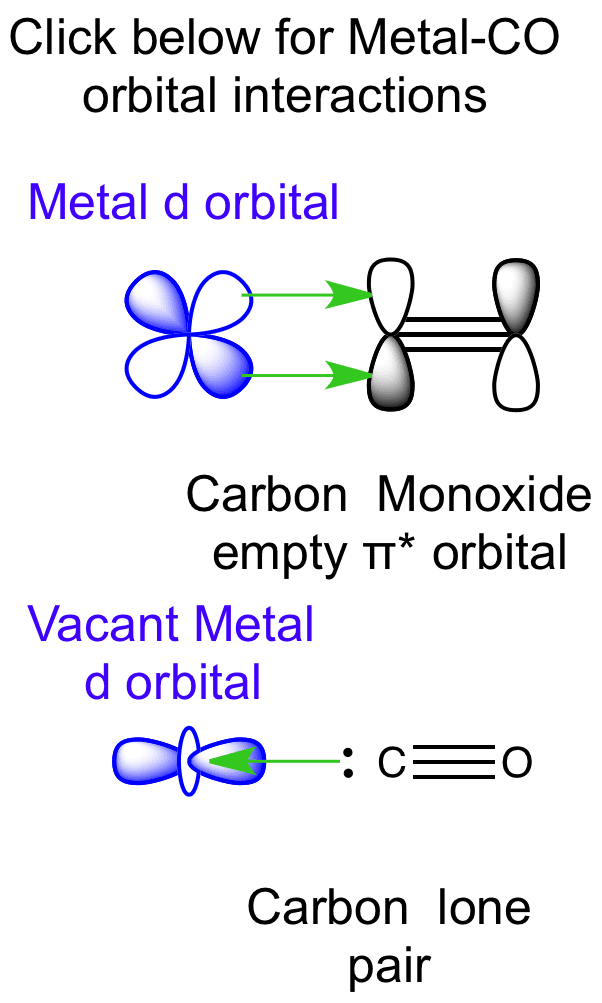
PDF Valence BondDescription of the CO ligand Molecular Orbital Description of the CO Ligand The CO LUMO orbitals are antibonding of * symmetry. These are empty orbitalsand canaccept electron density from a metal centre via ‐ backbonding with the metald(xy), d(xz)andd(yz), orbitals The CO HOMO orbital is a bonding orbital of symmetry with significant electron density on the carbon.
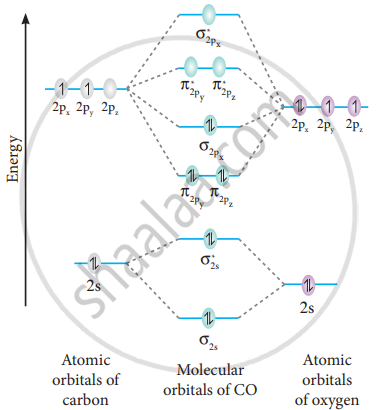
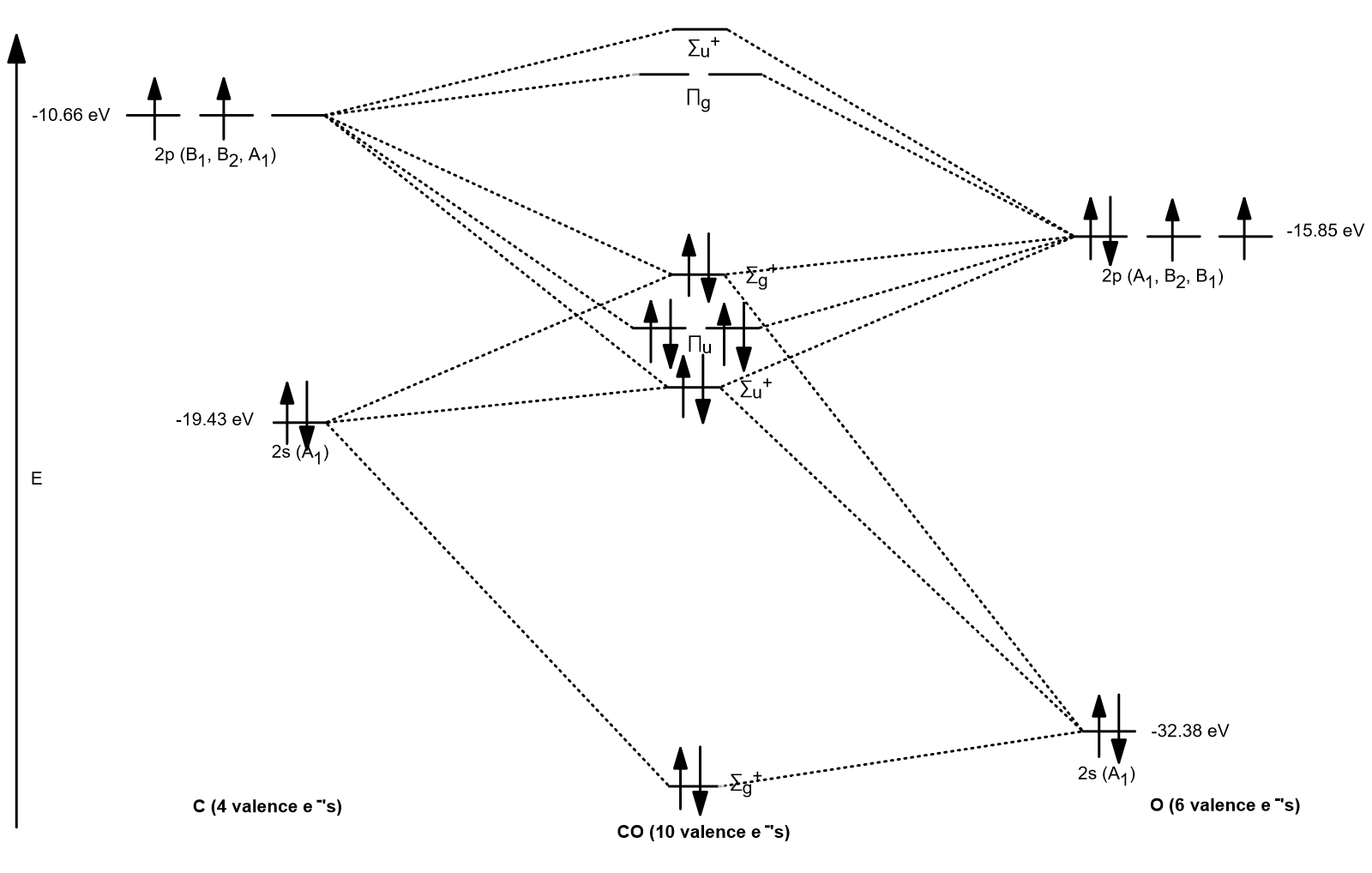
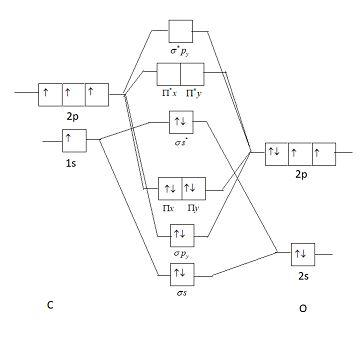
What is the bond order of CO? - Quora Answer (1 of 14): Molecular orbital energy level diagram of CO molecule can be given as. CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).According to molecular orbital diagram, molecular orbital configuration is given as σ2s² σ*2s² πx² πy² σ...
Give the orbital diagram for an atom of Co. | Study.com Give the orbital diagram for an atom of Co C o . Orbital Diagrams: Orbital diagrams can be used to represent the electronic configuration of an atom. The orbital diagram is a pictorial...
What is the molecular orbital energy diagram of CO? - Quora Answer (1 of 4):
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
orbitals - How to rationalise with MO theory that CO is a ... The electrons in the frontier orbital(s) play a special role for the chemical reactivity. In $\ce{CO}$, the HOMO is the $ 5 \sigma $ orbital (ref: your diagram), and has mainly $\ce{C}$ character. The two electrons in it act like a lone pair on the carbon.
Sulfur(S) electron configuration and orbital diagram Sulfur (S) excited state electron configuration and orbital diagram. This electron configuration shows that the last shell of the sulfur atom has six unpaired electrons (3s 1 3p x1 3p y1 3p z1 3d xy1 3d yz1 ). So the valency of sulfur is 6. From the above information, we can say that sulfur exhibits variable valency.
Molecular orbitals in Carbon Monoxide - ChemTube3D Orbital-orbital Interactions and Symmetry Adapted Linear Combinations; ... Molecular orbitals in Carbon Monoxide. CONTROLS > Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine …
CO Lewis Structure, Geometry, and Hybridization ... 09/03/2022 · Molecular Orbital Diagram of Carbon Monoxide (CO) The above image shows energy levels for the molecular orbitals of the carbon monoxide (CO) The molecular orbital diagram is a diagrammatic representation of showing …
Molecular Orbital diagram for CO - Ultraviolet and Visible ... Energy levels: Molecular Orbital Theory - revision 11:10. Molecular Orbital diagram for CO 5:09. Taught By. Patrick J O'Malley, D.Sc. Reader. Try the Course for Free. Transcript. Explore our Catalog Join for free and get personalized recommendations, updates and offers. ...
Carbon Monoxide Molecular Orbital Diagram Explanation A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. . combinations such as CO and NO show that the 3σg MO is higher in energy. Mulliken came up with theory known as Molecular Orbital Theory to explain questions like above.
Cobalt Electron Configuration (Co) with Orbital Diagram Orbital Diagram of Cobalt. The history of the Cobalt chemical element is quite rich in itself as it is one of the oldest chemical elements in the world. There is evidence that states the usage of this element as early as in the period of 1300 or so.
bonding in benzene - sp2 hybridisation and delocalisation An orbital model for the benzene structure. Building the orbital model. Benzene is built from hydrogen atoms (1s 1) and carbon atoms (1s 2 2s 2 2p x 1 2p y 1).. Each carbon atom has to join to three other atoms (one hydrogen and two carbons) and doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s 2 pair into …
how to draw molecular orbital diagram of co - Earn A Lot ... Molecular orbital diagram of N 2 is shown below. Molecular orbitals in Carbon Monoxide. Draw the orbital diagram for the ion Co2. The MO diagram for CO is. AO2s AO2s σ2s σ 2s strong head-on overlap AO2px AO2px π2px π 2px weak sidelong overlap AO2py AO2py π2py π 2py weak sidelong overlap AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus ...
How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
inorganic chemistry - Molecular orbital diagram of CO and ... Molecular orbital diagram of CO and charge localisation. Ask Question Asked 1 year, 9 months ago. Active 1 year, 9 months ago. Viewed 138 times 4 1 $\begingroup$ MO diagram of CO. My question concerns the interpretation of the Molecular Orbital of CO. I think I find it clear how you build it but I have some concerns about how you rationalize it.
What is the electron configuration of "Co"^"3+"? | Socratic The electron configuration of "Co"^(3+) is ["Ar"] 4s 3d^5. "Co" is in Period 4 of the Periodic Table, and "Ar" is the preceding noble gas. Cobalt is also in Group 9, so it must have 9 valence electrons. The valence shell configuration is therefore 4s^2 3d^7, and the core notation is bb"Co": ["Ar"] 4s^2 3d^7 When a transition metal forms an ion, the s electrons are removed before the d electrons.




![Synthesis of an Oxygen-Carrying [Co(salen)]2 Complex ...](https://cloudfront.jove.com/files/ftp_upload/10430/10430fig4v4.jpg)











![Molecular orbitals diagrams of [Co(NH3)6]3+](https://image.slidesharecdn.com/molecularorbitalsdiagramsofconh3631-211121141527/95/molecular-orbitals-diagrams-of-conh363-2-638.jpg?cb=1637504464)

Comments
Post a Comment