43 o2 molecular orbital diagram
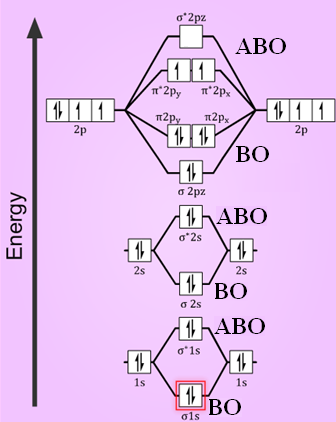
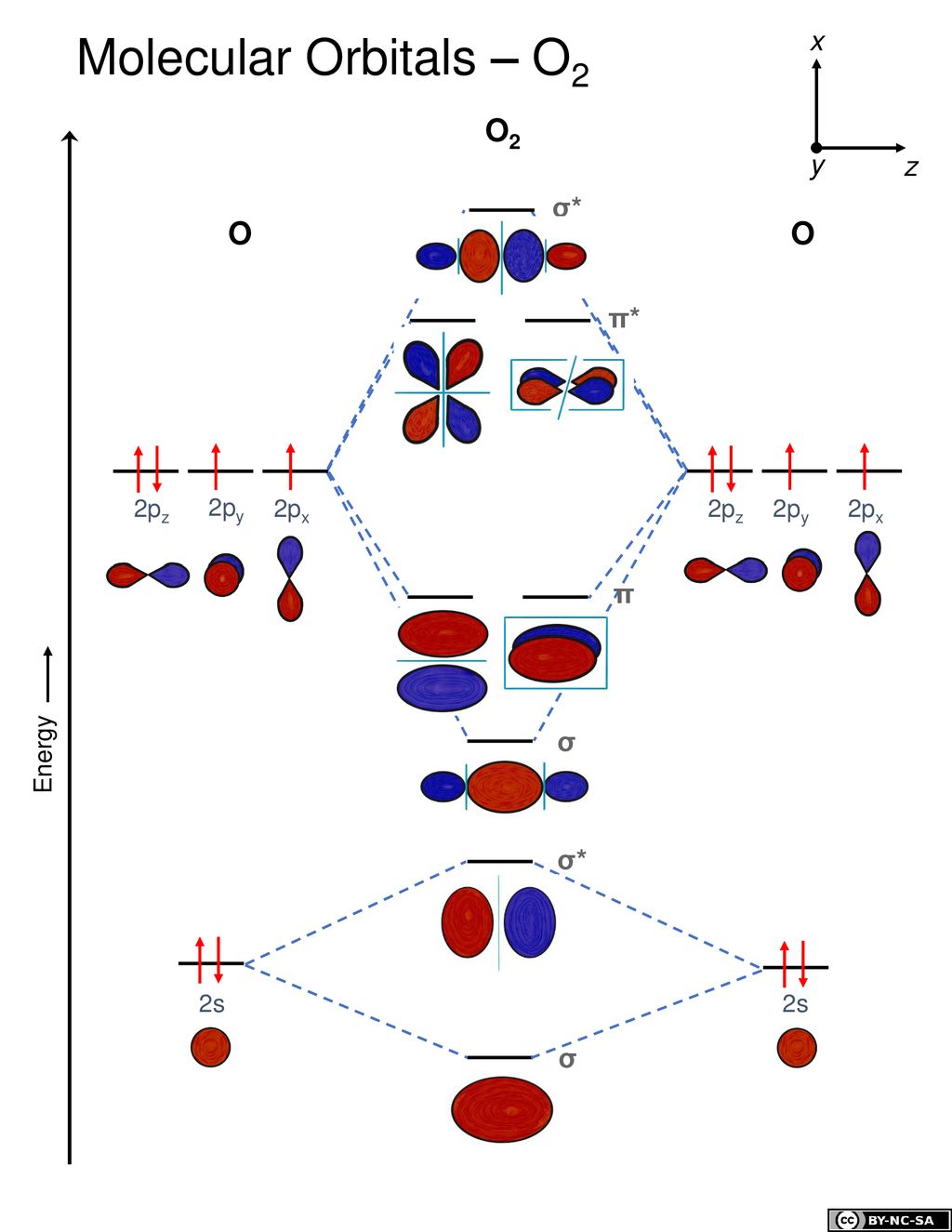
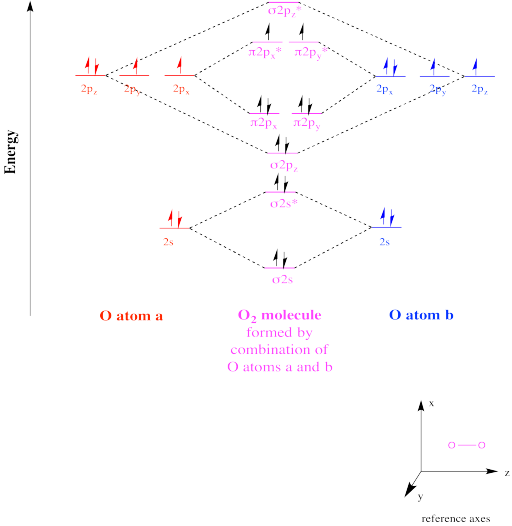
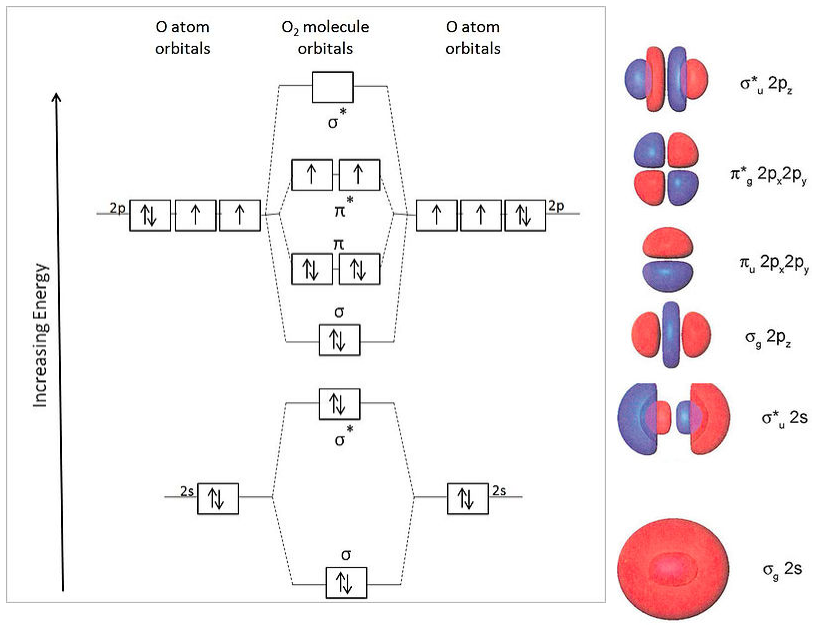
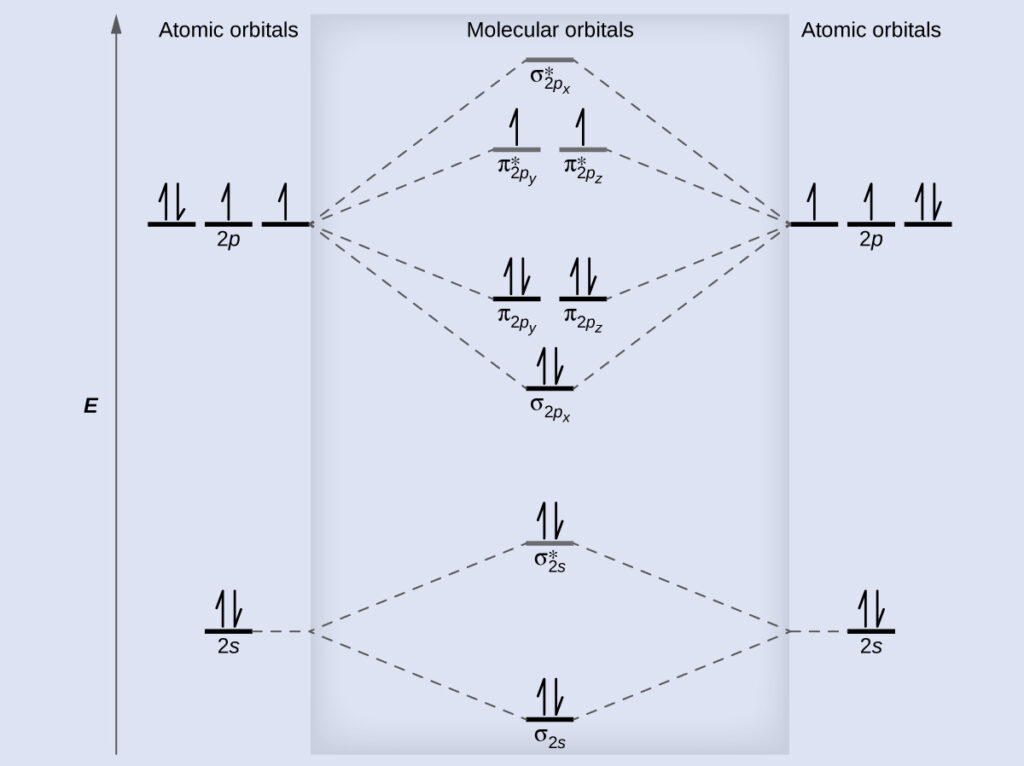
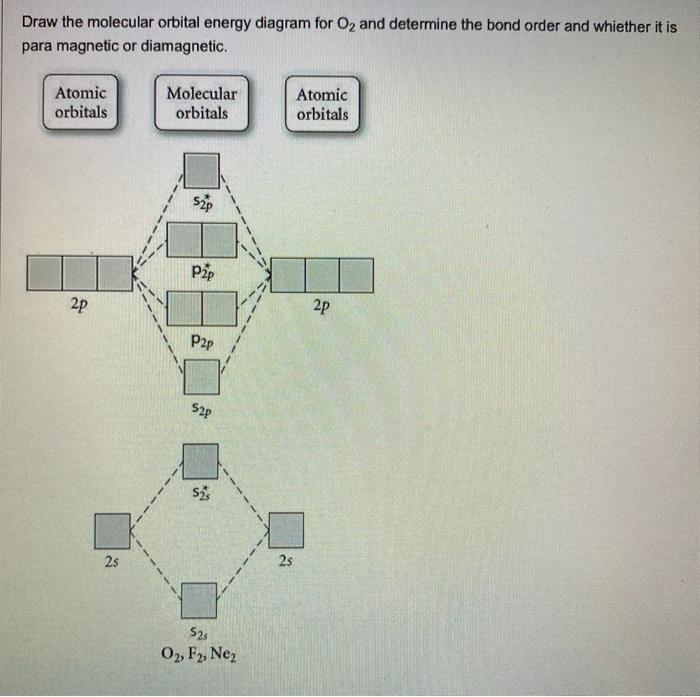
Bond order ofO2 O2+ O2 and O22 is in order A O2 langle ... Hint: First draw a molecular orbital diagram (MOT) where the atomic orbitals combine to form molecular orbitals. The total electrons associated with the molecules are filled in the MOT diagram. To solve this question, we need to write the molecular orbital configuration. To find out the bond order from the molecular orbital configuration is: 7.7 Molecular Orbital Theory - Chemistry Fundamentals Draw the molecular orbital diagram for the oxygen molecule, [latex]\ce{O2}[/latex]. From this diagram, calculate the bond order for [latex]\ce{O2}[/latex]. How does this diagram account for the paramagnetism of [latex]\ce{O2}[/latex]? Show Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 7.7.12.
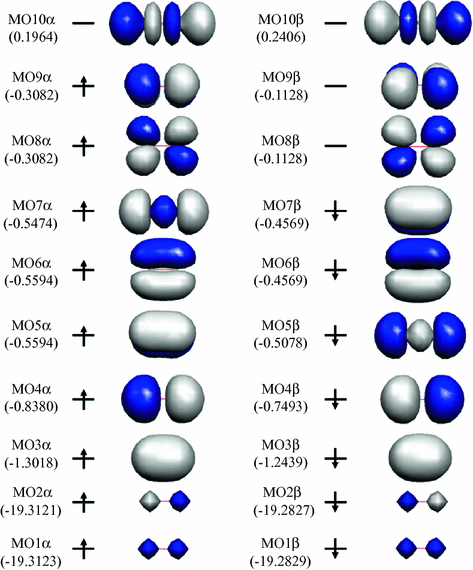
Draw the molecular orbital energy diagram for oxygen ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...
O2 molecular orbital diagram
Molecular Orbital Theory - Chemistry Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . Each oxygen atom contributes six electrons, so the diagram appears as shown in . MOLECULAR ORBITAL DIAGRAM OF O2, 02+AND O2(2-). - YouTube In this video, you will study about Molecular Orbital diagram of O2, O2+, O2(2-). We will also calculate the Bond order in each case and also the magnetic be... O2 Lewis Structure, Molecular Geometry, and Hybridization ... Feb 25, 2022 · The molecular orbital diagram shows the energy state at each level where the excited state increases from the bottom to the top. The left-hand side diagram is of O2 at ground level whereas the right-hand side diagram is of rearranged electrons as per the Lewis structure within the O2 molecule.
O2 molecular orbital diagram. Explain the formation of O2 molecule using molecular orbital ... The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. Explain the formation of O2 molecule using molecular class ... We know that Oxygen has atomic number = 8. Thus, the electronic configuration for an atom of oxygen in the ground state can be given as - $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as - Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... Molecular Orbital (MO) Diagram for O2(2+) - YouTube Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare...
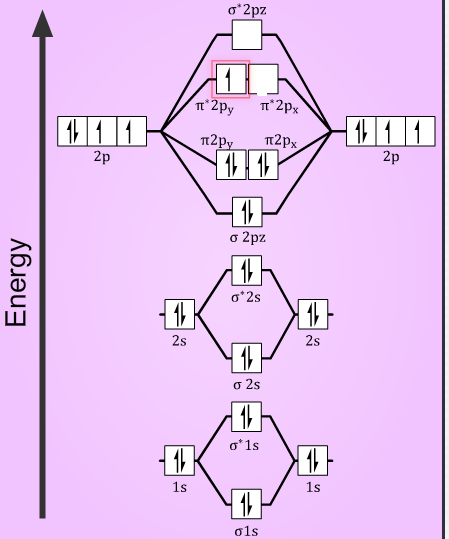
MO Diagram of O2 (Molecular Orbitals, Oxygen) - YouTube How to draw the molecular orbital diagram of O2. Sorry, the Sigma-1-s orbital is a little off-screen. Rest assured, it's filled. I wouldn't violate the au... Molecular Orbital Theory - Purdue University Electrons are added to molecular orbitals, one at a time, starting with the lowest energy molecular orbital. The two electrons associated with a pair of hydrogen atoms are placed in the lowest energy, or bonding, molecular orbital, as shown in the figure below. This diagram suggests that the energy of an H 2 molecule is lower than that of a ... MO DIAGRAM O2+ , O2 2+ ,O2- ,O2 2- (preparation of gate ... Follow me on instagram- me on facebook page- ... 45 b2 2- molecular orbital diagram - Modern Wiring Diagram The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels According to molecular orbital theory,molecular orbital diagram for helium molecule can be given as. From molecular orbital configuration,bond... B2 2- molecular orbital diagram
Chemical and structural origin of lattice ... - Nature Energy 25-03-2019 · b, Schematic formation of O NB by extrapolating the molecular orbital energy diagram for octahedral MO 6. E F represents the Fermi level. c , d , Charge density difference ( c ) and projected DOS ... By writing molecular orbital configuration for NO,CO,O2 ... Mar 18, 2018 · "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram. Study Guide and Solutions Manual to ... - Academia.edu Study Guide and Solutions Manual to Accompany T.W. Graham Solomons / Craig B. Fryhle / Scott A. Snyder / Jon Antilla In the molecular orbital diagram for O2^ + ion, the ... In the molecular orbital diagram for O 2+ ion, the highest occupied orbital is: A σ MO orbital B π MO orbital C π ∗ MO orbital D σ ∗ MO orbital Medium Solution Verified by Toppr Correct option is C) As it can be seen from the given structures that in the molecular orbital diagram for O 2+ ion, the highest occupied orbital is π ∗ MO orbital.
Draw a molecular orbital diagram of N2 or O2 with magnetic ... Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means.
Draw molecular orbital diagram of O2 or N2 with magnetic ... Click here👆to get an answer to your question ️ Draw molecular orbital diagram of O2 or N2 with magnetic behavior and bond order.
Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
8 - Drawing Molecular Orbital Diagrams — Flux Science Well, s-p mixing doesn't occur with diatomic oxygen, creating a molecular orbital diagram like the first in this article. This is because, as more electrons are added to a system, the higher the energy becomes, due to their electrostatic repulsion. If the energy of the 2s and 2p orbitals are too far apart, mixing won't occur.
Molecular Orbital (MO) Diagram for O2(-) - YouTube When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p) orbitals. This different from Nitrogen, where it's the othe...
Electronic valence molecular orbital configuration of "O ... You'll need the molecular orbital (MO) diagram of "O"_2. Begin with the atomic orbitals. Oxygen atom has 2s and 2p valence orbitals and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get "O"_2. Two 2s orbitals combine to give a sigma_(2s) bonding and sigma_(2s)^"*" antibonding MO.
Singlet oxygen - Wikipedia Molecular orbital diagram of two singlet excited states as well as the triplet ground state of molecular dioxygen. From left to right, the diagrams are for: 1 Δ g singlet oxygen (first excited state), 1 Σ + g singlet oxygen (second excited state), and 3 Σ − g triplet oxygen (ground state).
How many molecular orbitals are in o2 ... What is the molecular orbital diagram for O2 2? O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbitals. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals.
Inorganic Chemistry | Vol 61, No 5 - pubs.acs.org 07-02-2022 · The crystal structures of two low-symmetry novel titanium phosphates [NH4TiP2O7 (P21/c) and TiP2O7 (P1̅)] were solved via ab initio methods from powder X-ray diffraction data. The low symmetry of the new compounds requires a combination of various techniques, spectroscopic and diffraction, to complete the structural puzzle, revealing a unique three …
Feynman diagrams - Overleaf, Online LaTeX Editor TikZ-Feynman is a LaTeX package allowing Feynman diagrams to be easily generated within LaTeX with minimal user instructions and without the need of external programs.It builds upon the TikZ package and its graph drawing algorithms in order to automate the placement of many vertices.TikZ-Feynman still allows fine-tuned placement of vertices so that even complex …
O2 Lewis Structure, Molecular Geometry, and Hybridization ... Feb 25, 2022 · The molecular orbital diagram shows the energy state at each level where the excited state increases from the bottom to the top. The left-hand side diagram is of O2 at ground level whereas the right-hand side diagram is of rearranged electrons as per the Lewis structure within the O2 molecule.
MOLECULAR ORBITAL DIAGRAM OF O2, 02+AND O2(2-). - YouTube In this video, you will study about Molecular Orbital diagram of O2, O2+, O2(2-). We will also calculate the Bond order in each case and also the magnetic be...
Molecular Orbital Theory - Chemistry Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . Each oxygen atom contributes six electrons, so the diagram appears as shown in .



























Comments
Post a Comment