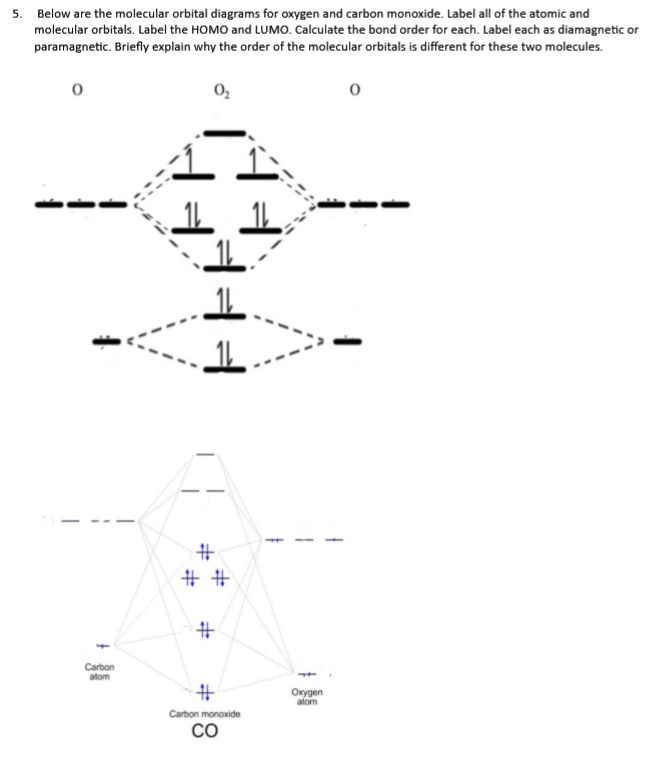
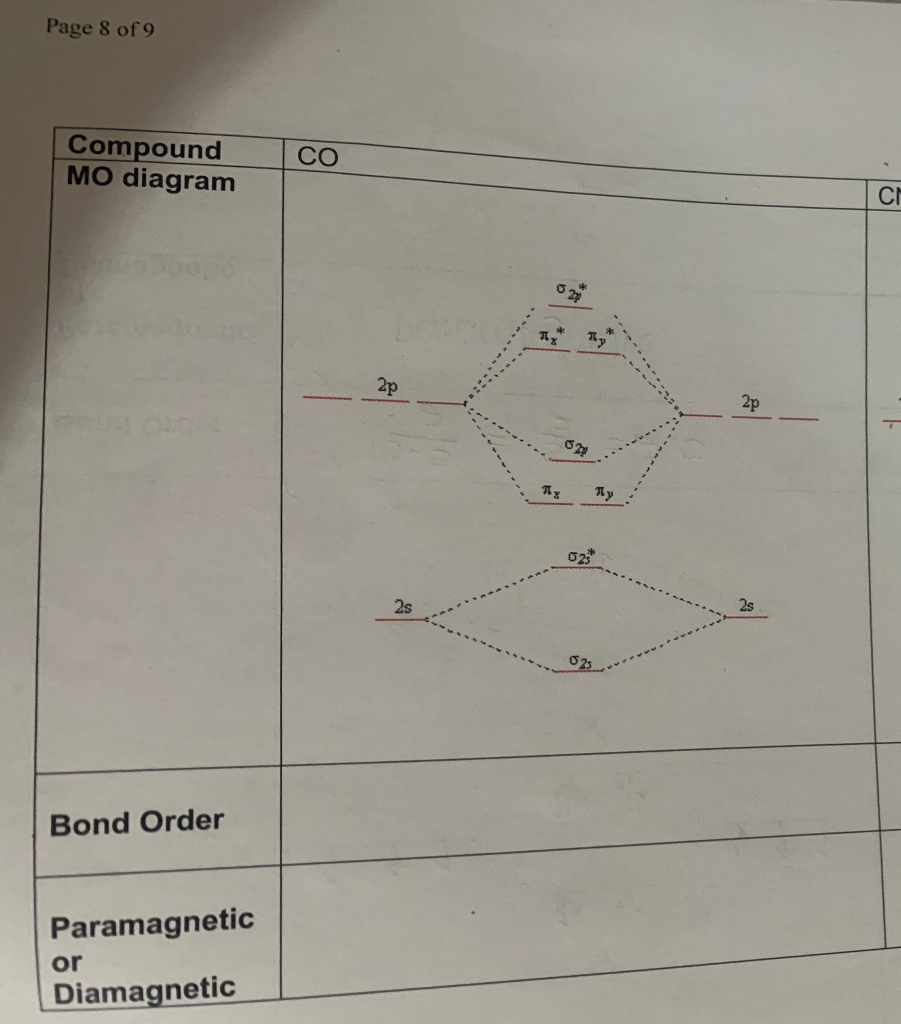
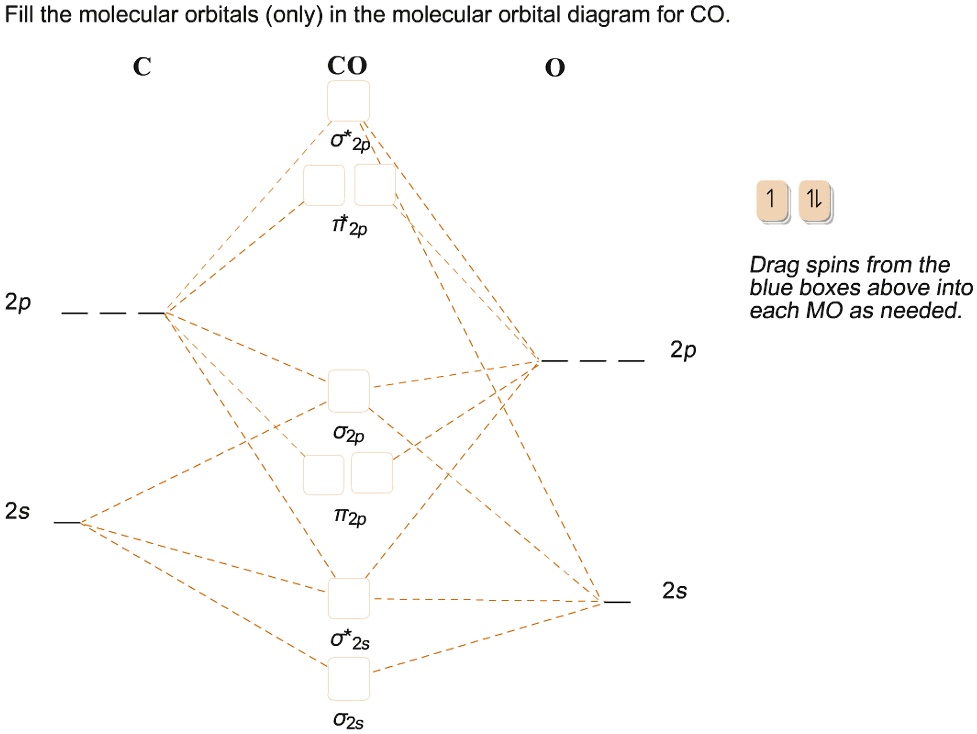
39 mo diagram for co
Cyanide Molecular Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. In this answer of Martin's, you can find a molecular orbital diagram of $\ce{CO}$. 5.3.1: Polar bonds - Chemistry LibreTexts 4 days ago — Carbon monoxide MO diagram. Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The ...
PDF Thermodynamic Assessment of the Co-Mo System - NIST Co-Mo system and the investigative techniques used. The last column indicates whether the measured values were used in the assessment. 2.1 Phase Diagram Data The Co-Mo phase diagram is shown in Fig. 1. This system contains total of eight phases: four solution phases: liquid, cph (Co), fcc (Co), and a bcc (Mo) terminal solid solutions, plus
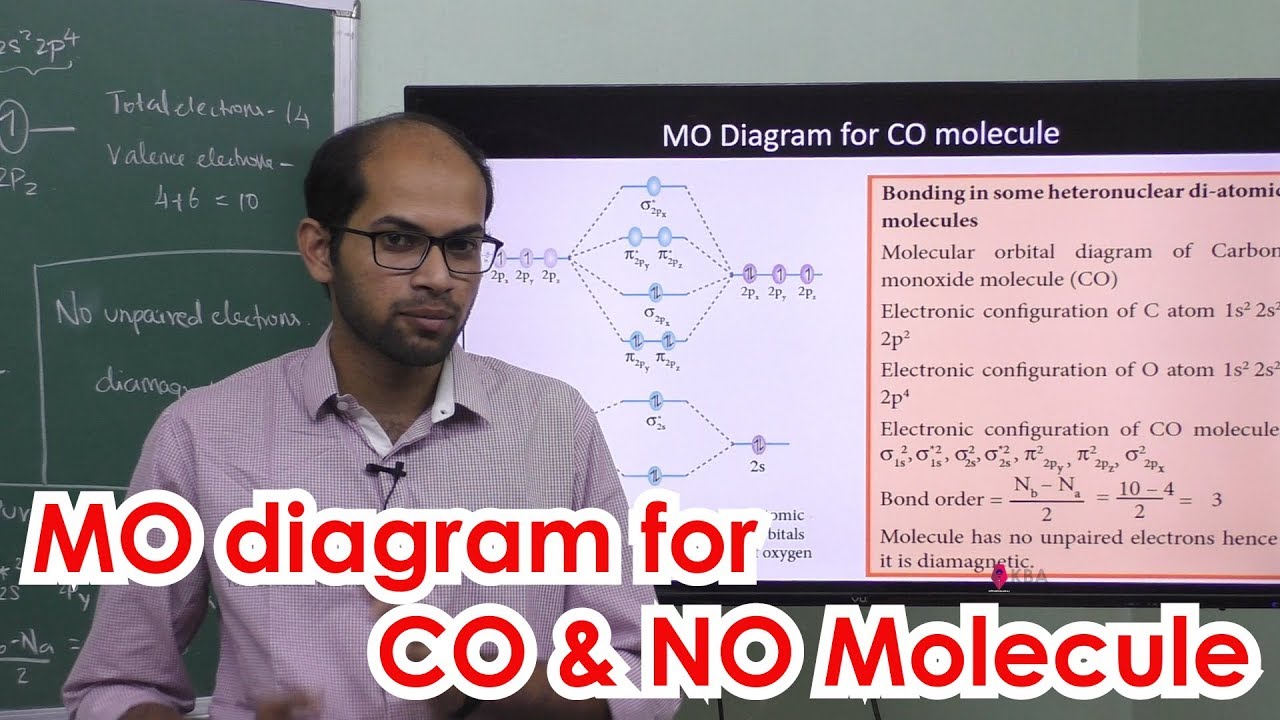
Mo diagram for co
Explain the MO diagram for NO molecule. - Sarthaks eConnect | Largest Online ... 1 Answer. 1. Electronic configuration of N atom is 1s2 2s2 2p3. 2. Electronic configuration of O atom is 1s2 2s2 2p4. 3. Electronic configuration of NO molecule is σ1s2 σ*1s2 σ2s2 σ*2s2 π2px2 π2py2 π2pz2 π*2px1. 4. Bond order = N b−N a 2 N b − N a 2 = 10−5 2 10 − 5 2 = 2.5. MO diagram of CO - The Student Room It can be difficult to determine which is bonding, anti-bonding and non-bonding when you have a lot of mixing take place. Just judging from an accurate MO diagram is sufficient, here I'd say the bonding orbitals are 1 sigma and 2 x 1 pi whilst the remaining occupied sigma orbitals are non-bonding giving us the familiar bond order of 3. 0. Molecular orbital energy diagram of CO+ - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Mo diagram for co. orbitals - How to rationalise with MO theory that CO is a two-electron donor ... C O is isoelectronic with N X 2. Sketch MO diagrams for C O and N X 2. Point out key differences between the diagrams and use the diagram to explain why C O acts as a two-electron donor through carbon rather than through oxygen. Solved 1. Draw the MO diagram of CO (3 pts) 2. Sketch the | Chegg.com Chemistry questions and answers. 1. Draw the MO diagram of CO (3 pts) 2. Sketch the LUMO of CO (2 pts) 3. Explain why both LUMO and HOMO of CO are located on the carbon end (2 pts) Question: 1. Draw the MO diagram of CO (3 pts) 2. Sketch the LUMO of CO (2 pts) 3. What is the molecular orbital energy diagram of CO? - Quora Answer (1 of 4): Molecular Orbital Diagram of CO | All About Chemistry Molecular Orbital Diagram of CO. TAGS; Molecular Orbital Diagram; Previous article Wohl-Ziegler Bromination. Next article Molecular Orbital Diagram of NO. All About Chemistry. . Hello Reader! Thanking for reading this post, If you find it to be informative, pls share it and visit our website.
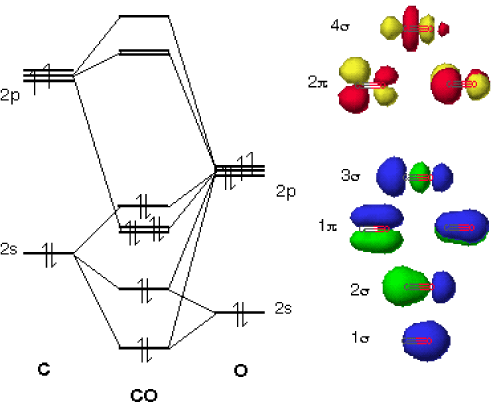
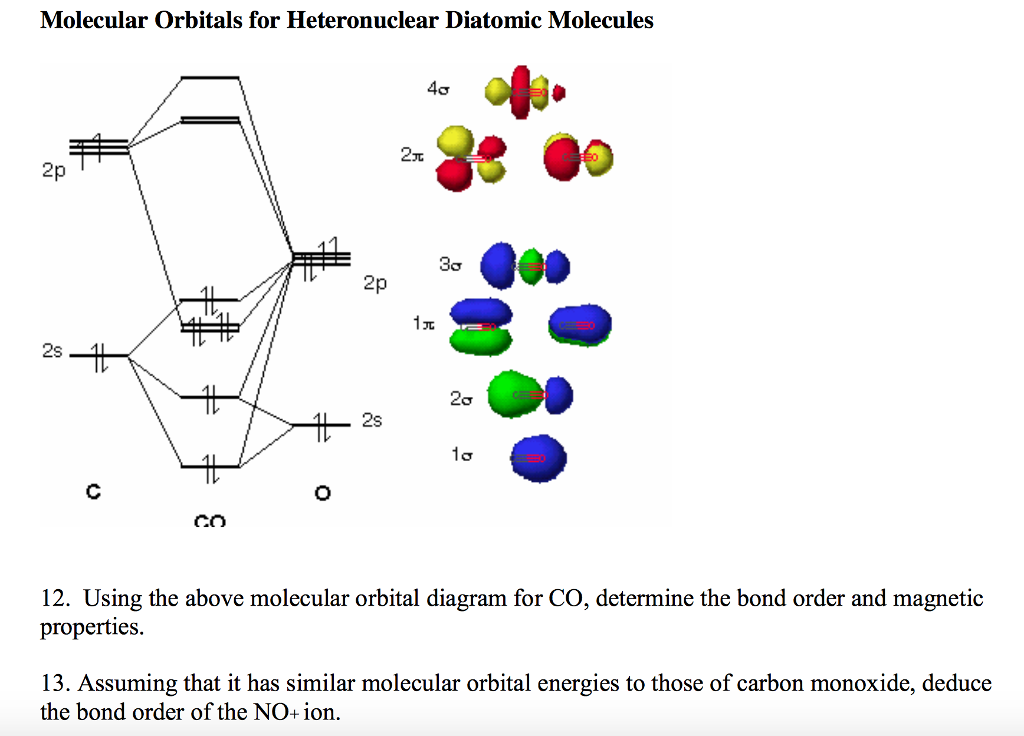
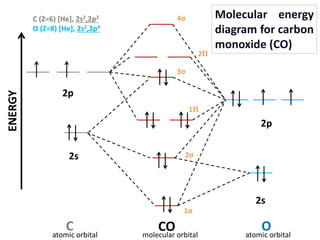
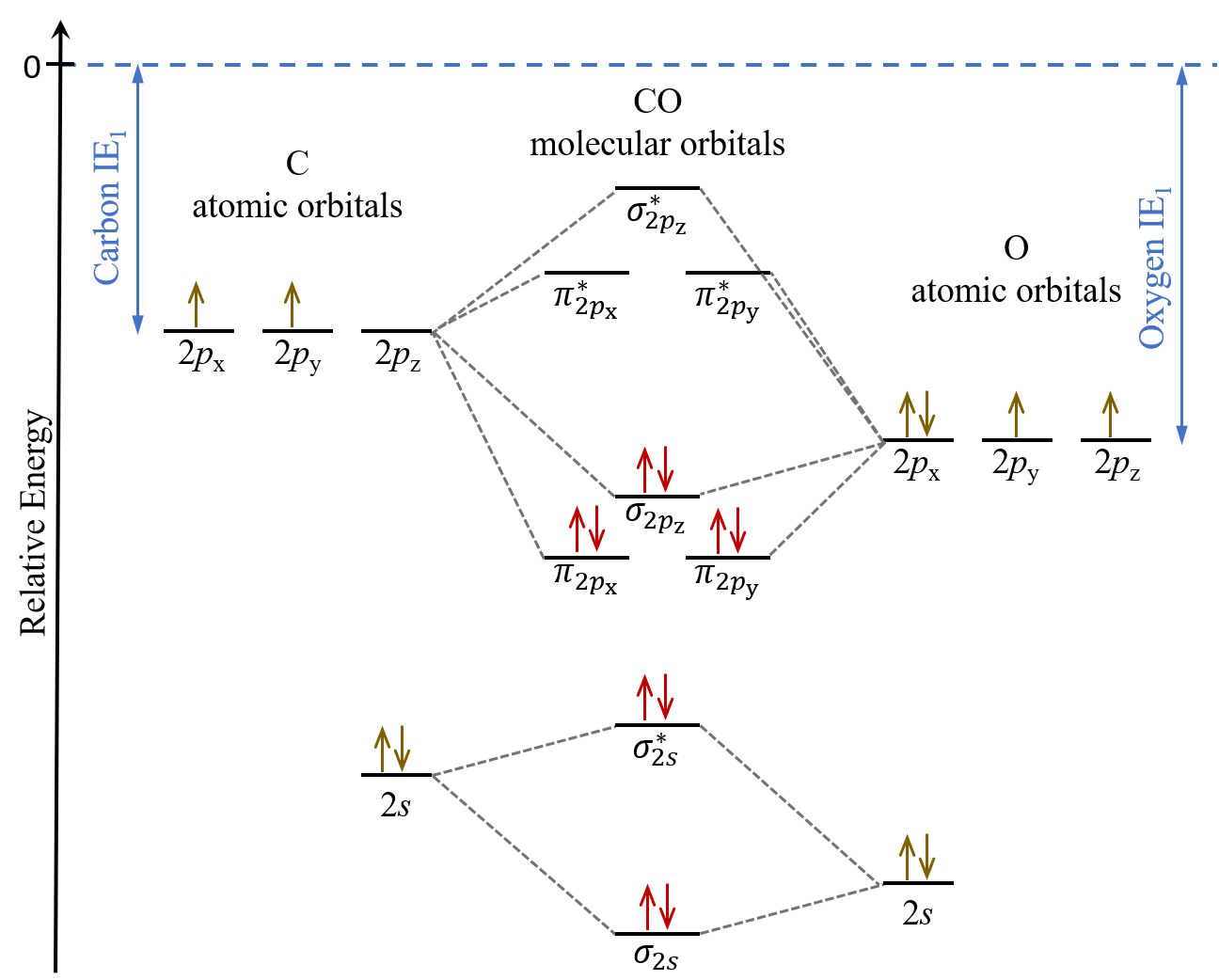
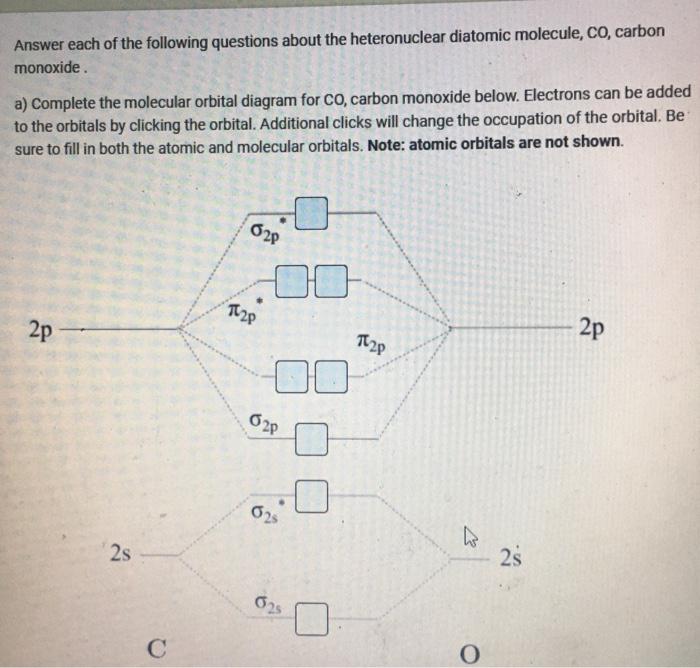
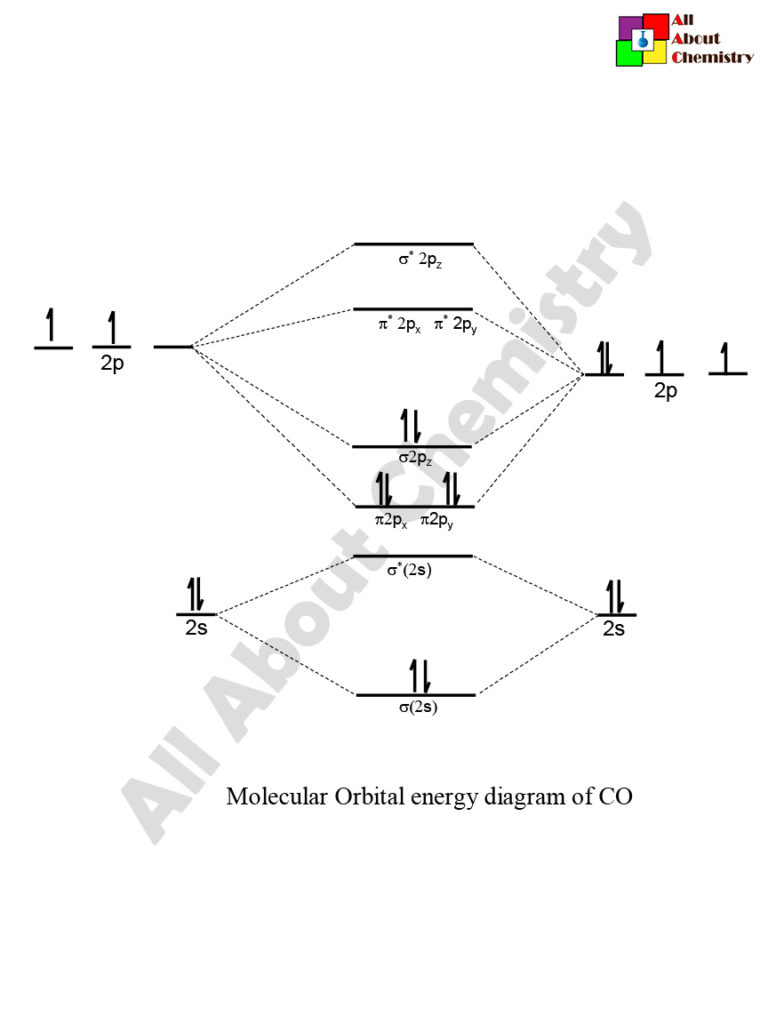
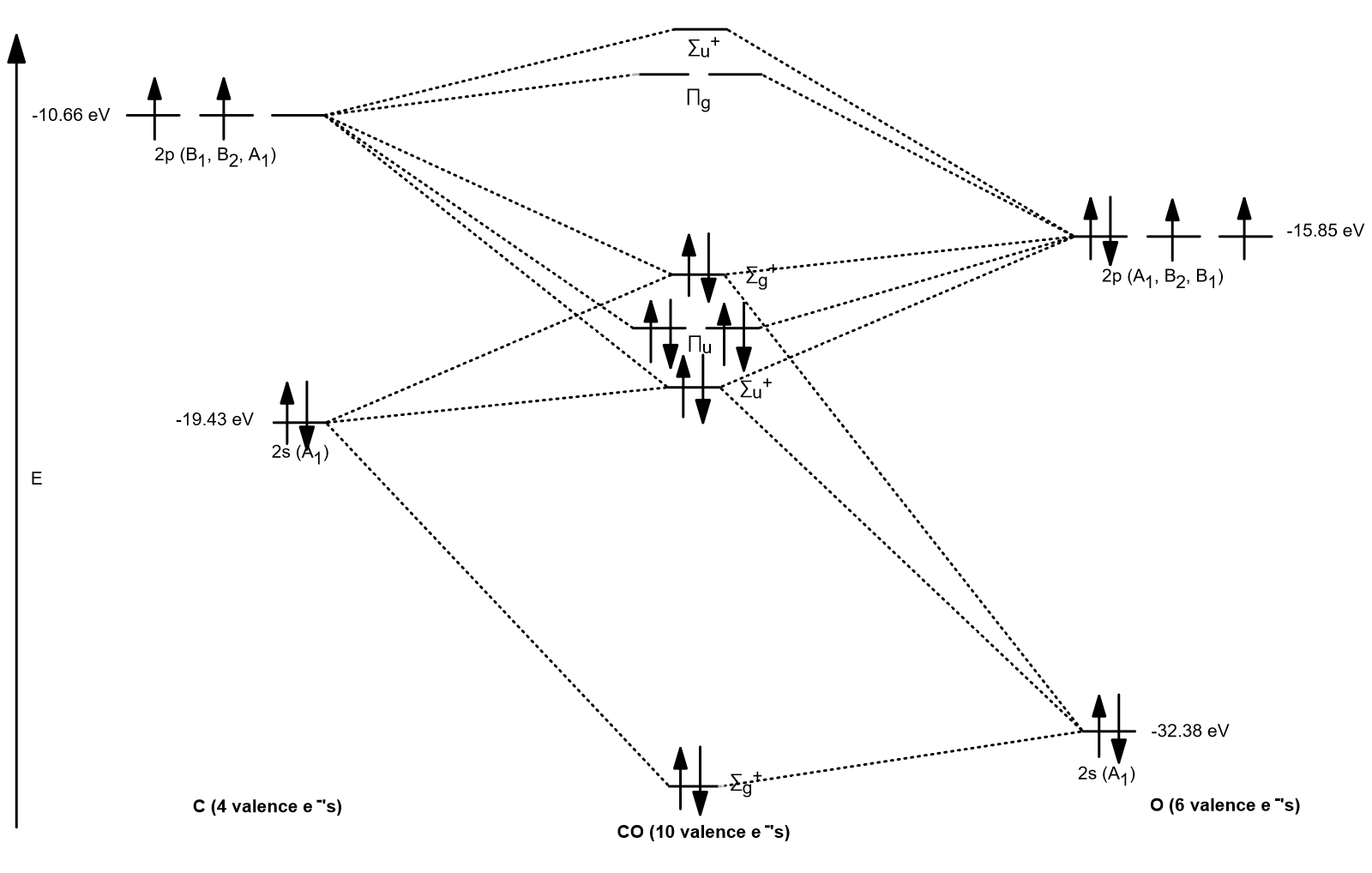
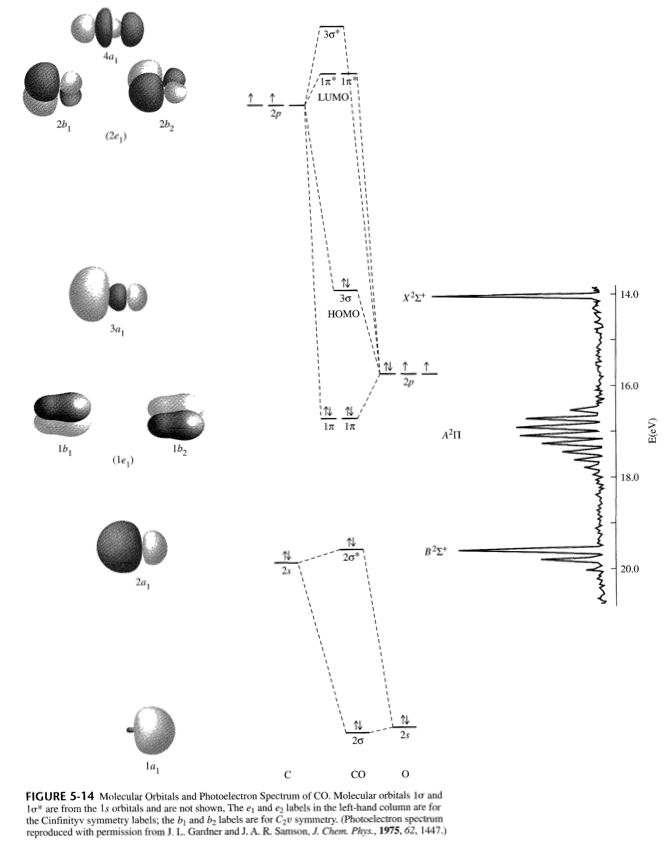
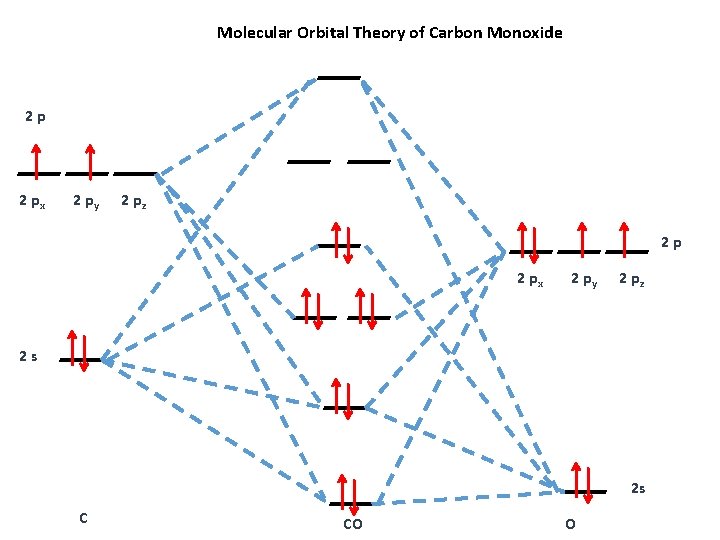
Carbon Oxides The molecular orbital diagram of carbon monoxide is very similar to that of molecular nitrogen. Carbon, with 4 valence electrons, and oxygen with 6 valence ... PDF MO Diagrams for Diatomic Molecules Heteronuclear Diatomic Molecules: CO In molecules with more than one type of atom, MOs are formed from AOs that have different energies. Consider CO: 2sa 2pa C 2sb 2pb C≡O O σ σ* π π* σ σ* Bonding orbitals get polarized towards oxygen Anti-bonding orbitals get polarized towards carbon HOMO is on carbon LUMO is on carbon too! Heterodiatomic Molecules: MO diagram for CO Example: CO This diagram is based on calculations and comparison to (But it is not drawn exactly, just approximately.) about the sp-mixing and the shapes and sizes of MOs. Notice that the bonding orbitals are bigger on the oxygen and the antibonding orbitals are bigger on the carbon. We will talk more about the consequences of this later. MO diagram and Lewis Structure of CO - CHEMISTRY COMMUNITY For bond order it is a good rule of thumb that triple bonds = 3 double = 2 and single = 1 but if you want to be sure, I personally like drawing a quick MO diagram sketch on the side and do the formula (bonding-antibonding)/2 to find the bond order if I am not certain about a bond. Top 8 posts • Page 1 of 1
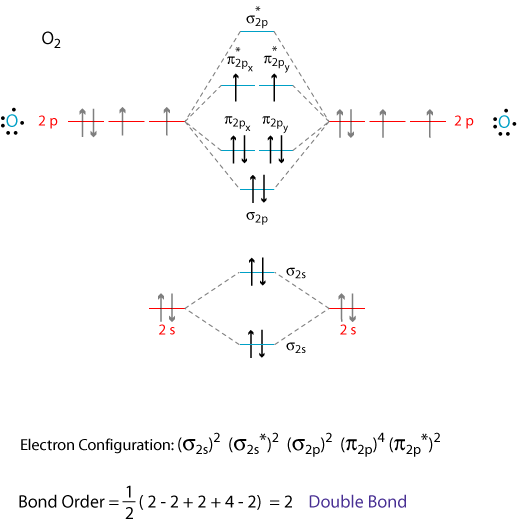
By writing molecular orbital configuration for NO,CO,O2 molecules ... - Socratic.org "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram. PDF Bonding in transition metal complexes Example: Constructing a MO diagram for Chromium Hexacarbonyl, Cr(CO) 6 Cr ππππ-bonding AOs T2g:(3dxy,3dxz,3dyz) T1u:(4px,4py,4pz) • T2g previouslyconsiderednon-bondingin σ-bondingscheme • T1ucombineswith T1u SALCin in σ-bondingscheme • T1g, T2u π-SALCs are non-bonding Cr non-bonding AOs T2g: (3 dxy, 3dxz, 3dyz) Cr σσσσ-bonding ... Molecular Orbital diagram for CO - Ultraviolet and Visible Spectroscopy | Coursera Molecular Orbital diagram for CO Introduction to Molecular Spectroscopy University of Manchester 4.7 (2,077 ratings) | 43K Students Enrolled Enroll for Free This Course Video Transcript The course introduces the three key spectroscopic methods used by chemists and biochemists to analyse the molecular and electronic structure of atoms and molecules. Molecular orbitals in Carbon Monoxide - ChemTube3D Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry ...
MO Diagrams - GitHub Pages First step is to determine which MO diagram we're using. In this case, we're using the standard one. Draw out the MO diagram and label in the valence electrons. Boron has 2 electrons in the 2s 2 s orbitals and 1 electron in the 2p 2 p orbital. That's it for the MO diagram of B2 B 2! To check, count how many electrons there are in total.
Draw MO diagram of CO and calculate its bond order | Class 11 Chemistry ... Draw MO diagram of CO and calculate its bond order .Welcome to Doubtnut. Doubtnut is World's Biggest Platform for Video Solutions of Physics, Chemistry, Math...
PDF Example: Constructing a only MO diagram for Iron Pentacarbonyy,l, Fe(()CO)5 Example: Constructing a MO for Chromium Hexacarbonyy,l, Cr(()CO) 6 O h E 8C 3 6C 2 6C 4 3C 2 i 6S 4 8S 6 3 h 6 d /h h =48 12 0 0 0 -400 0 0 0 A 1g 12 0 0 0 -12 0 0 0 0 0 0 0 A 2g 12000-120000 0 0 0 E g 24 0 0 0 -24 0 0 0 0 0 0 0 T 1g 360 001200000481 T 2g 360 001200000481 A 1u 120 00-120000 0 0 0 A 2u 120 00-120000 0 0 0 E u 24 0 0 0 -24 0 0 0 0 0 0 0 T 1u 360 0012000120481 T 2u 360 0012000120481
PDF MO Diagrams for More Complex Molecules MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
Molecular Orbitals for Carbon Monoxide Molecular Orbitals for CO. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital in the energy level correlation diagram shown The results displayed may be switched between those from a low level of calculation and those from a high level.
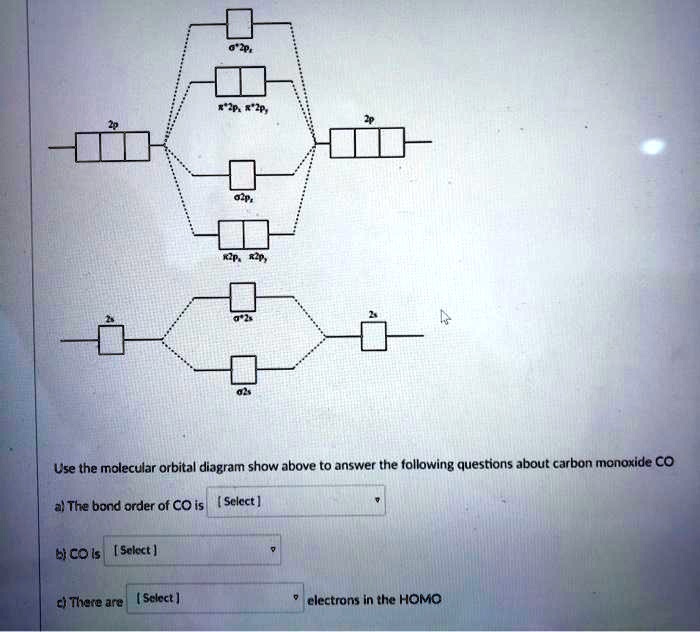
What is the bond order of CO? - Quora Molecular orbital energy level diagram of CO molecule can be given as. CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).According to molecular orbital diagram, molecular orbital configuration is given as σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰ Thus , bond order = 1/2 (8-2)=3 82.7K views View upvotes
Carbon Monoxide Molecular Orbital Diagram Explanation May 09, 2018 · A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not.. combinations such as CO and NO show that the 3σg MO is higher in energy. Mulliken came up with theory known as Molecular Orbital Theory to explain questions like above. According to Molecular .
Solved 1- Draw the molecular orbital diagram for MO theory | Chegg.com Chemistry questions and answers. 1- Draw the molecular orbital diagram for MO theory of [Co (CO)6]3+ . And show why it is a n acceptor ligand. 2 - Draw the molecular orbital diagram for MO of [CONH3]3+ why NH3 Molecule is called a sigma donor ligand. 3- Draw the molecular orbital diagram for MO of [CoF6]3-.
PDF Lecture 3 - chem.tamu.edu Figure 3-1 Molecular orbitals of Cr(CO) 6 (Only interactions between Ligand (σ- and π*) orbitals and metal d-orbitals are shown.) Simplified MO energy level diagram for Cr(CO) 6. Note the empty π* orbitals. Only three are involved in overlap with metal d orbitals.
Draw MO diagram of CO and calculate its bond order. - Sarthaks eConnect | Largest ... Draw MO diagram of CO and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 17, 2020 by Maisa (45.8k points) selected Dec 18, 2020 by Panna01 . Best answer. 1. Electronic configuration of C atom: 1s 2 2s 2 2p 2. ...
inorganic chemistry - Molecular orbital diagram of CO and charge localisation ... MO diagram of CO. My question concerns the interpretation of the Molecular Orbital of CO. I think I find it clear how you build it but I have some concerns about how you rationalize it. Particularly, from what I read the reason we envision carbon as being partially negatively charged in CO is that the HOMO of the molecule lies closer to the ...
Molecular orbital energy diagram of CO+ - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
MO diagram of CO - The Student Room It can be difficult to determine which is bonding, anti-bonding and non-bonding when you have a lot of mixing take place. Just judging from an accurate MO diagram is sufficient, here I'd say the bonding orbitals are 1 sigma and 2 x 1 pi whilst the remaining occupied sigma orbitals are non-bonding giving us the familiar bond order of 3. 0.
Explain the MO diagram for NO molecule. - Sarthaks eConnect | Largest Online ... 1 Answer. 1. Electronic configuration of N atom is 1s2 2s2 2p3. 2. Electronic configuration of O atom is 1s2 2s2 2p4. 3. Electronic configuration of NO molecule is σ1s2 σ*1s2 σ2s2 σ*2s2 π2px2 π2py2 π2pz2 π*2px1. 4. Bond order = N b−N a 2 N b − N a 2 = 10−5 2 10 − 5 2 = 2.5.



![PDF] Bond Order and Chemical Properties of BF, CO, and N2 ...](https://d3i71xaburhd42.cloudfront.net/32b08acb98b762ef91be755b59e48a5514d2b628/2-Figure4-1.png)

![Exchange coupling through diamagnetic [Fe(CO) 4 ] 2 ...](https://pubs.rsc.org/image/article/2011/DT/c0dt01221a/c0dt01221a-f2.gif)







Comments
Post a Comment